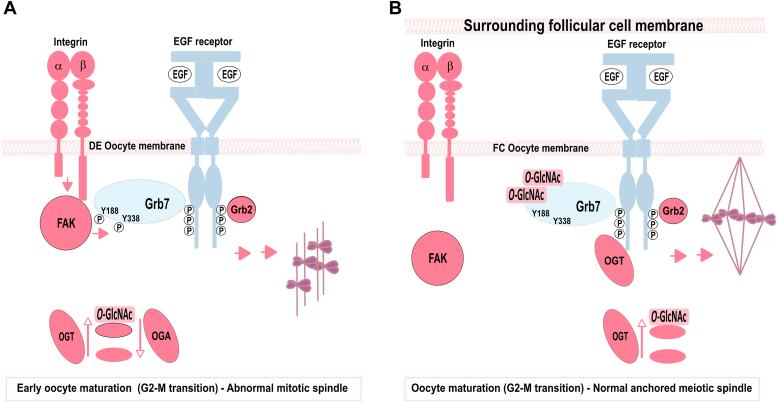
Figure 10.
Model for Xenopus oocyte protection by follicular cells from abnormal maturation (G2–M transition) triggered by an epidermal growth factor (EGF) receptor (EGFR)/integrin–FAK complex and the adaptor Grb7 phosphorylation.A, in defolliculated oocytes (DE), EGF addition to EGFR-expressing oocytes triggers the tyrosine kinase receptors autophosphorylation (P) and the recruitment of effectors including adaptors Grb2 and Grb7. Activated EGFRs form heterocomplexes with integrin–FAK, leading to Grb7 phosphorylation on two tyrosine residues Y188 and Y338. Consequently, these signaling pathways favor a rapid meiotic transition from prophase I to metaphase II (maturation) and abnormal spindle formation that does not localize at the oocyte plasma membrane. B, in follicular cells (FCs), the formation of a multicomplex between EGFR–EGF and integrin–FAK is prevented by the layer of surrounding follicular cells and their tight contact with the oocyte membrane. Phosphorylated EGFRs recruit OGT, Grb7 is O-GlcNAcylated, and abnormal oocyte phenotype is avoided. The oocyte OGT–OGA balance avoids unwanted phenotypic defaults. In A and B, inhibition of protein O-GlcNAcylation (OGT inhibitor) increases phenotypic defaults. Folliculated oocytes are more insensitive toward protein O-GlcNAcylation removal (OGA inhibition). Oocyte endogenous proteins are in red, and heterologous expressed proteins are in blue. FAK, focal adhesion kinase; OGT, O-GlcNAc transferase.