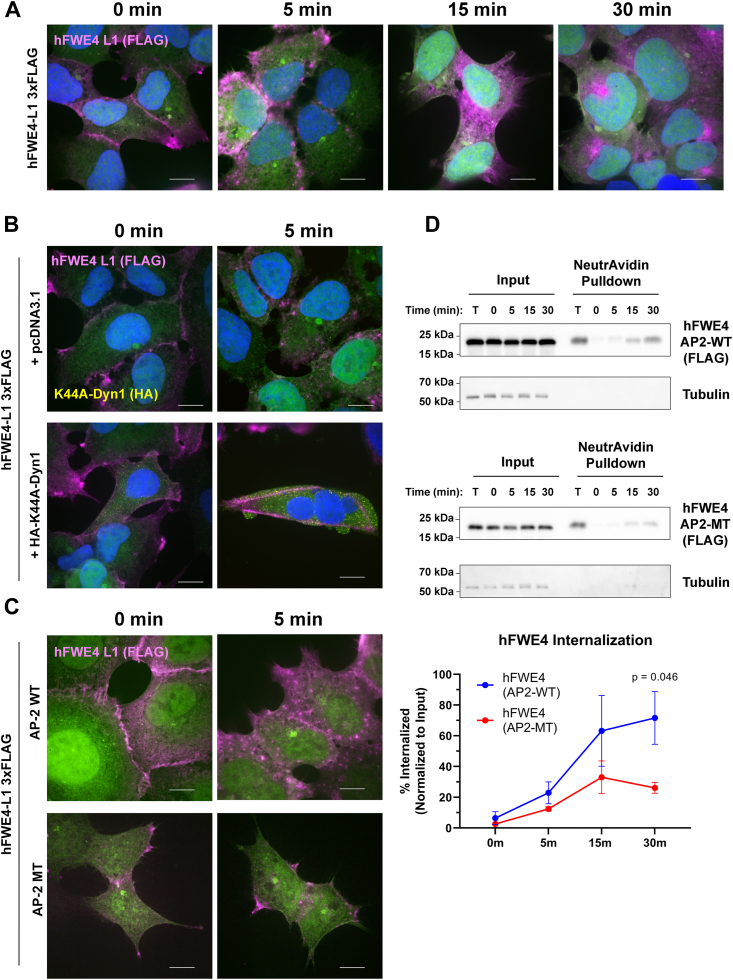
Figure 6.
Rapid internalization of hFWE4 from the plasma membrane is dependent on functional AP-2 motifs and dynamin1-mediated vesicle scission.A, HEK293 stably coexpressing non-fused EGFP (green) and hFWE4-L1 3xFLAG were pulsed with FLAG antibody for 45 min on ice, washed, chased for 0, 5, 15 or 30 min at 37 °C, and then fixed for fluorescent tyramide based detection (magenta) and Hoechst nuclear labeling (blue). B, HEK293 stably coexpressing non-fused EGFP and hFWE4-L1 3xFLAG were transfected with either pcDNA3.1 or Dyn1-K44A-HA prior to antibody pulse and 0 or 5 min chase as described above. Following tyramide development, Dyn1-K44A-HA was labeled with anti-HA (yellow). C, HEK293 stably coexpressing non-fused EGFP and either wild-type hFWE4-L1 3xFLAG, or AP-2 motif mutant hFWE4-L1 3xFLAG were pulsed with FLAG antibody and chased for 0 or 5 min before tyramide based detection as described above. A−C, single Z-slices from representative 100× confocal imaging for native EGFP (green), immunolabeled hFWE4 (magenta) at indicated time points from each, scale bar = 10 μm. D, representative immunoblots from cell surface biotinylation and internalization assays performed in AP2-WT (top panels) and AP-2MT (bottom panels) hFWE4 expressing HEK293 cells. T = unstripped samples representing total surface biotinylated protein; 0 min = samples subject to Mesna stripping immediately following biotinylation; 5 to 30 min = samples chased at 37 °C for indicated time points to allow internalization, then Mesna stripped to remove remaining biotin from protein remaining at the cell surface. Tubulin was used as a cytosolic control. Input-normalized FLAG signal at each chase timepoint was compared to total surface (T) signal as a readout of the fraction of internalized hFWE4. Graph presents densitometric data from N = 3 replicates, statistical significance was determined using a two-way ANOVA with post-hoc Holm-Sidak correction).