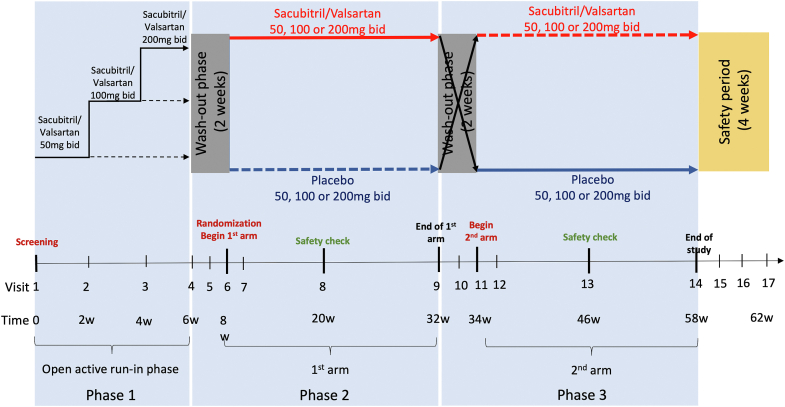
Figure 1.
Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor vs Placebo in Patients With Congenital Systemic Right Ventricular Heart Failure Trial (PARACYS-RV) study schema. This figure provides an overview of the study design. Phase 1 includes the screening visit and the open active run-in phase of 6 weeks. Phase 2 begins by a 2-week washout period; then, patients are randomized in the first arm of 24 weeks of active therapy or the corresponding placebo. Phase 3 is the mirror of the second phase; each patient receives the active therapy or the corresponding placebo, depending on phase 2 after a 2-week washout period. Safety monitoring is evaluated at a halfway time point for each treatment arm. A safety period of 4 weeks ends the study. bid, twice daily.