Table 1. Evaluations of Conditions for the Reaction of 1a and 2a to Afford 3aa.
| entry | 2a (equiv) | CF3SO3H (equiv) | time (min) | yield of 3a (%)b | yield of 4a (%)b |
|---|---|---|---|---|---|
| 1 | 3.0 | 0.45 | 60 | 70 | 0 |
| 150 | 94 | 6 | |||
| 2c | 3.0 | 0.45 | 60 | 60 | 0 |
| 150 | 94 | 6 | |||
| 3c | 3.0 | 0.45 | 82 | 40 | 1 |
| 147 | 69 | 3 | |||
| 4 | 3.0 | 0.20 | 60 | 46 | 0 |
| 5 | 3.0 | 0.10 | 60 | 39 | 0 |
| 6 | 3.0 | 0.05 | 60 | 32 | 0 |
| 7 | 3.0 | 0.01 | 60 | 31 | 0 |
| 120 | 60 | 0 | |||
| 240 | 94 | 6 | |||
| 8 | 1.0 | 0.01 | 48 | 45 | 2 |
| 75 | 78 | 5 | |||
| 9d | 1.0 | 0.01 | 48 | 14 | 0 |
| 75 | 24 | 1 | |||
| 10e | 3.0 | 0.001 | 30 | 18 | 0 |
| 100 | 38 | 0 | |||
| 11e | 2.0 | 0.001 | 30 | 23 | 0 |
| 100 | 58 | 1 | |||
| 12e | 1.5 | 0.001 | 30 | 29 | 2 |
| 100 | 88 | 2 | |||
| 13e | 1.1 | 0.001 | 30 | 30 | 0 |
| 100 | 90 | 2 |
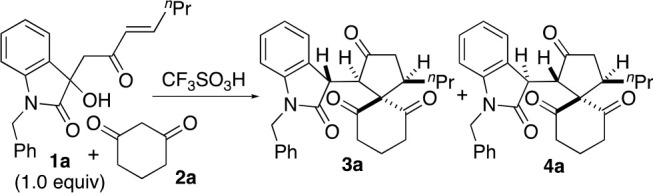
For the reactions, to a solution of 2a (indicated equivalents relative to 1a) and CF3SO3H (indicated equivalents relative to 1a) in CDCl3 (1.0 mL), 1a (0.029 mmol, 1.0 equiv) was added, and the mixture was heated at 60 °C. See the Supporting Information for details.
Determined by 1H NMR analysis.
To a solution of 1a (0.029 mmol, 1.0 equiv) and 2a (indicated equivalents) in CDCl3 (1.0 mL) (entry 2) or in C6D5CD3 (1.0 mL) (entry 3), CF3SO3H (indicated equivalents) was added, and the mixture was heated at 60 °C.
2b (2.0 equiv) was added.
A 0.11 mmol scale reaction.

