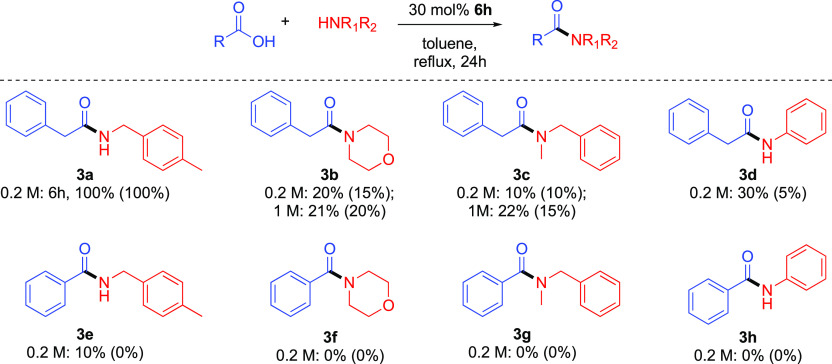
Figure 5.
Substrate scope of catalytic amidation with bromosilanol 6h at 0.2 or 1.0 M concentration in both acid and amine with isolated yields after acid–base workup and dry column vacuum chromatography. The figures in parentheses are isolated yield of a background reaction without catalyst, where the amide products were pure after acid–base workup. Amide 3a precipitated from the reaction mixture on cooling and could be collected by filtration. Amide 3d was triturated with n-hexane to remove unreacted aniline.