TABLE 2: Chemical structure of substituted derivatives with noteworthy activity against Cryptococcus neoformans and Cryptococcus gattii strains obtained by applying molecular modification.
| Starting material (prototype) | Derivative with increased activity | Reference |
|---|---|---|
 |
 |
|
| 109 | ||
| 2-hydroxynaphthalene-1,4-dione | 1 H -cyclopenta[ b ]naphtho[2,3- d ]furan-5,10(3a H,10b H )-dione | |
 |
 |
108 |
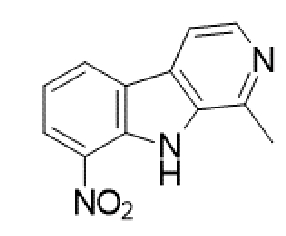
| 9 H -pyrido[3,4- b ]indole | 1-methyl-8-nitro-9 H -pyrido[3,4- b ]indole | |
 |
 |
111 |
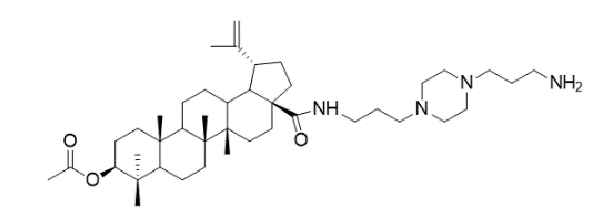
| Betulinic acid | ( 1R,3a S,5a S,5b R,9 S,1(1a R )-3a-((3-(4-(3aminopropyl)piperazin-1-yl)propryl)carbamoyl)5a,5b,8,8, 11a-pentamethyl-1-(prop-1-em-2-yl)-icosahydro-1 H -cyclopenta[ a ]chrysen-9-yl acetate | |
 |
 |
110 |
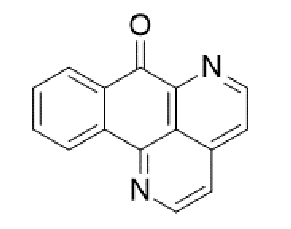
| 7 H -naphtho[1,2,3- ij ][2,7]naphthyridin-7-one | 3-ethylthieno[3',2':4,5]benzo[1,2 - d ]isoxazole-4,8-dione | |
 |
 |
112 |
| 5,7-dihydroxy-2-methyl-4 H -chromen-4-one | ( E )-2-(5-hydroxy-2-methyl-4-methylene-4,6,9,10-tetrahydrooxocino[3,2- g ]chromen-8-yl)ethyl acetate | |
 |
 |
113 |
| ( S )-6-isopeopyl-3-methylcyclohex-2-enone | 3-hydroxy-2-isopropyl-5-methylcyclohexa-2,5,-diene-1,4-dione | |
 |
 |
114 |
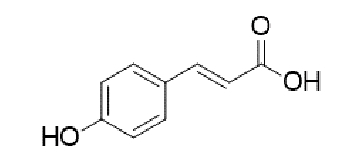
| ( E )-3-(4-hydroxyphenyl)acrylic acid | ( E )-ethyl 3-(4-hydroxyphenyl)acrylate | |
 |
 |
105 |
| 5-methyl-5 H -indolo[3,2- b ]quinolin-11(10 H )-one | 5,10-dimethyl-5 H -indolo[3,2- b ]quinolin-11(10 H )-one |
Structures were designed using Chemdraw 19.0
