Abstract
Facial attractiveness plays important roles in social interaction. Electrophysiological and neuroimaging studies found several brain areas to be differentially responsive to attractive relative to unattractive faces. However, little is known about the time course of the information processing, especially under the unattended condition. Based on a “cross-modal delayed response” paradigm, the present study aimed to explore the automatic mechanism of facial attractiveness processing of females with different physiological cycles and males, respectively, through recording the event-related potentials in response to (un)attractive opposite-sex faces by two experiments. The attractiveness-related visual mismatch negativity (attractiveness vMMN) in posterior scalp distribution was recorded in both the experiments, which indicated that attractive faces could be processed automatically. And high-attractive opposite-sex faces can elicit larger vMMN in males than females in menstrual period in Study 1, but similar as females in ovulatory period in Study 2. Furthermore, by comparison, the latency of attractiveness vMMN in females with the ovulatory period was the longest. These results indicated as follows: (1) Males were more sensitive to attractive female faces, (2) females in ovulatory period were also attracted by the attractive male faces, (3) the long vMMN latency in females during ovulatory period suggested a special reproductive motivation to avoid being tainted by genes, which takes priority over the breeding motivation.
Keywords: facial attractiveness, automatic processing, event-related potential (ERP), visual mismatch negativity (vMMN), female in ovulatory period, female in menstrual period
Instruction
In evolutionary psychology, preference for attractive faces serves an adaptive function. Individuals with attractive faces are more preferred in mate selection because facial attractiveness is considered to be a symbol of health, sound immunity, and reproductive advantages (Perrett, 2012; Fink & Penton-Voak, 2002; Rhodes & Zebrowitz, 2002). Attractive people have, on average, better parasite resistance (Buss, 2005; Kościński, 2008), greater physical and reproductive fitness (Malo et al., 2009; Preston, Stevenson, Pemberton, Coltman, & Wilson, 2003), longevity (Henderson & Anglin, 2003), easier shedding of genetic load (Kokko, Brooks, Jennions, & Morley, 2003), higher intelligence (Buss, 2015; Li et al., 2013), and better mental health (Smith et al., 2009). The perception of facial attractiveness would help people select a high-quality mate and transmit their genes to the succeeding generation (Fink & Penton-Voak, 2002; Gallup & Frederick, 2010; Rhodes, 2006; Rhodes, Morley, & Simmons, 2013). This initial screening process should be most relevant at the very early stages of mate selection, before any significant investment is made. Thus, the perception of the facial attractiveness is adaptive. It is an evolved disposition assisting an individual in choosing a mate with good genes, thereby leading to fitness gain in his or her offspring.
A large number of empirical studies found that individuals are born with an innate preference for attractive faces (Duuren, Kendell-Scott, & Stark, 2003; Hahn et al., 2016; Hahn & Perrett, 2014; Slater, Quinn, Hayes, & Brown, 2000), and the perception of facial attractiveness shows many similarities across cultures (Hoss, Ramsey, Griffin, & Langlois, 2005; Langlois et al., 2000; Rhodes et al., 2001; Rhodes et al., 2005) and ages (Bronstad & Russell, 2007). More importantly, some studies suggest that facial attractiveness is also prioritized by our attention system, in addition to threatening stimuli. Its early stages can occur preattentively (Palermo & Rhodes, 2007) in specialized brain areas such as the fusiform face area, lateral occipital cortex (Chatterjee, Thomas, Smith, & Aguirre, 2009), and right orbitofrontal cortex (Tsukiura & Cabeza, 2011). In addition, brain regions related to reward and positive emotion can be automatically activated by attractive faces, including sublenticular extended amygdala at the basilar part of forebrain, ventral tegmental area (VTA), nucleus accumbens, and orbitofrontal cortex (Chatterjee et al., 2009; Ishai, 2007; Kranz & Ishai, 2006; O’Doherty et al., 2003; Winston, O’Doherty, Kilner, Perrett, & Dolan, 2007).
Recent event-related potential (ERP) research addressed the meaning and characteristics of facial attractiveness and the biases (e.g., sexual, age, and racial) of judging attractiveness. Several related ERP components have been found. One is the classic N170 that reflects the neural processing of facial structural encoding (Compton, 2003; Gajewski, Schlegel, & Stoerig, 2009; Marzi & Viggiano, 2010). The second is the anterior P2 (120–220 ms), suggesting a fast attentional bias to attractive opposite-sex faces (Hooff, Crawford, & Vugt, 2011). The other ERP components include the early posterior negativity (EPN; 230-280ms), N300 (around 300 ms) and so on (Pizzagalli et al., 2002; Werheid, Schacht, & Sommer, 2007; Zhang & Deng, 2012; Zhang et al., 2011). It can be seen that researchers have made some progress in examining the intracerebral dynamic time interval change in processing facial attractiveness in conscious way, showing the differences elicited by attractive and unattractive faces in early and late stages. Generally, the most attractive faces elicited the largest brain responses (ERP amplitude), and the least attractive faces elicited the smallest ones (Hooff et al., 2011; Marzi & Viggiano, 2010; Schacht, Werheid, & Sommer, 2008; Werheid et al., 2007; Zhang et al., 2011).
But most of these experiments were conducted in an attended condition. As discussed above, the perception of facial attractiveness should be occurred automatically at the very early stages of attention, before any significant cognitive investment is made. So it is highly valuable to study the automatic mechanism of facial attractiveness in unattended condition. In order to fill up this gap, a classic ERP component, visual mismatch negativity (vMMN), the negative ERP component measured at the temporo-occipital electrodes with variable latency between 150 and 350 ms after stimulus onset, was investigated in this study. vMMN can be generated under unattended conditions and is considered as an index of automatic processing, which is derived by subtracting the ERPs elicited by standard stimuli (i.e., frequently repeated stimuli) from the ERPs elicited by deviant stimuli (i.e., infrequently repeated stimuli; Astikainen, Lillstrang, & Ruusuvirta, 2008; Berti, 2011; Czigler, Balázs, & Pató, 2004; Gábor, Motohiro, & István, 2011; Pazo-Alvarez, Amenedo, Lorenzo-López, & Cadaveira, 2004; Qiu et al., 2011; Stefanics, Kremláček, & Czigler, 2014b; Zhao & Li, 2006). To track the automatic processing, the “cross-modal delayed response” paradigm Wei et al., 2002, which offers a well-established means of assessing automaticity in the absence of attention, was used. The basic design involved the presentation of a series of pictures and tones in random order, hence the term “cross-modal.” During the current experiment, participants were asked to pay attention to the auditory stimuli (the attended modality) and ignore the visual stimuli (the unattended modality) and respond only after the imperative faint auditory signal occurred, hence the term “delayed response.” According to Wei, Chan, and Luo (2002), participants would fully engaged in the detection of the auditory signals and would find it difficult to pay attention to visual information; therefore, the more pure vMMN would be elicited (Wei et al., 2002). Based on these analyses, larger amplitude of vMMN responses to more attractive faces can be understood as the automatic allocation of more resources in preattention processing.
However, even though both sexes may have evolved to value facial attractiveness highly in mate choice, the specific features they are attracted to and the adaptive reasons involved are different (Li & Kenrick, 2006). Firstly, behavioral and neuroimaging studies showed sex differences in perception and processing of facial attractiveness in mate choice (Aharon et al., 2001; Cloutier, Heatherton, Whalen, & Kelley, 2008; Iaria, Fox, Waite, Aharon, & Barton, 2008; Kranz & Ishai, 2006; Penton-Voak, Jacobson, & Trivers, 2004; Senior, 2003; Yan, Wei, Zhao, Zheng, & Zhang, 2016). As people age, men’s fertility decreases relatively slowly over the life span, whereas women’s fertility decreases quickly after 30 years old and disappears by menopause (Li et al., 2013). So men may have evolved to prefer fertile and attractive partners more eagerly than women. A stronger activation in orbitofrontal cortex can be observed in males when judging attractive relative to unattractive faces, but this difference cannot be observed in females (Aharon et al., 2001; Boothroyd et al., 2017; Cloutier et al., 2008; Kampe, Frith, Dolan, & Frith, 2001; Kranz & Ishai, 2006; O’Doherty et al., 2003). In addition, males relative to females placed a greater emphasis on facial attractiveness when judging mate value (Buss & Schmitt, 1993; Li, Bailey, Kenrick, & Linsenmeier, 2002; Miner & Shackelford, 2010). Second, apart from discussing the features of attractive face owners, researchers found that the perceivers’ own hormone level and fertility also affect how they view the attractiveness of the opposite sex (Cárdenas & Harris, 2007; Jones, Vukovic, Little, Roberts, & Debruine, 2011; Little, Debruine, & Jones, 2011; Little, Jones, & Debruine, 2011; Montoya, Horton, & Kirchner, 2008; Oinonen & Mazmanian, 2007; Welling et al., 2007). For female individuals, the levels of estrogen and progesterone change with phases of the menstrual cycle. These hormones have various physiological effects and can act on the mind; hence the phase of the menstrual cycle may influence female’s perception of male faces (Kościński, 2008; Oinonen & Mazmanian, 2007). Comparing with females in menstrual phase (the infertile phase), females in ovulatory period (the fertile phase) show more preference for male faces with a relatively high attractiveness, holding that females in ovulatory period exhibit more stronger attention bias to attractive male faces (Cobey, Little, & Roberts, 2015; Duncan et al., 2007; Durante, Griskevicius, Simpson, Cantú, & Li, 2012; Gangestad & Thornhill, 1998; Hooff et al., 2011; Jones et al., 2005; Little, Jones, Burt, & Perrett, 2007; Maner, Gailliot, & Dewall, 2007; Pawlowski & Jasienska, 2005). According to good gene hypothesis (Thornhill, Gangestad, & Moller, 1999), attractive (masculinized and symmetric) male faces are related to mate quality. And females’ preference for attractive male faces changes with fecundity. That is, females in high fecundity phase would prefer attractive male faces (Conroybeam, Buss, Pham, & Shackelford, 2015; Gildersleeve, Haselton, & Fales, 2014; Jones et al., 2005; Oda, Okuda, Takeda, & Hiraishi, 2014; Pentonvoak et al., 1999; Penton-Voak & Perrett, 2000; Roberts & Little, 2008; Wang, Hahn, Fisher, Debruine, & Jones, 2014). Therefore, as described above, we should not examine the difference in mate choice criteria by looking for sex differences simply. It is necessary to distinguish between women’s different physiological cycles when comparing their preferences for attractive faces of the opposite sex.
As we know, few studies have examined the time course of automatic processing of attractive faces using the vMMN component. In particular, we do not know whether there are gender differences in processing highly attractive faces of the opposite sex automatically. Therefore, the first aim of the present study was to explore the automatic perception of the opposite-sex facial attractiveness under unattended condition. Additionally, given that female hormone levels vary in different stages of physiological cycle, is there same automatic processing over attractive opposite-sex faces during female menstrual period with a low hormone level and female ovulatory period with the highest hormone level? The second aim was to explore the different automatic mechanism of opposite-sex facial attractiveness among females in menstrual and ovulatory period and males. We designed two studies: In Study 1, menstruating females and males were selected as participants to investigate whether there are differences in processing highly attractive opposite-sex faces. In Study 2, ovulatory females and males were selected as participants to conduct this comparison. We predicted that (1) highly attractive opposite-sex faces would exhibit smaller vMMN in females in menstrual period (FMs) than in males, (2) when females in ovulatory period (FOs) and males were used as participants, their vMMN exhibited by highly attractive opposite-sex faces may show smaller or no difference.
Study 1
In Study 1, we examined the automatic processing of highly attractive opposite-sex faces in females in menstrual period and males.
Method
Participants
A total of 64 healthy right-handed (measured by the Reitan Test) undergraduate students (19–23 years; 32 FMs and 32 males were awarded extra course credit in exchange for their participations). The female participants were asked how many days have passed since onset of their last period of menses. When the participants were in the first to third day of their menstrual period, they were invited to participate in our experiments. Otherwise they were asked to wait till these days to come (Beall & Tracy, 2013). They all had normal or corrected-to-normal visual acuity and normal hearing. None of them reported any history of neurological or mental diseases. And their sexual orientations are all heterosexual. This study was carried out in accordance with the recommendations of Ethical Principles of Psychologists and Code of Conduct, The American Psychological Association. The protocol was approved by the Research Ethics Committee of Shandong Normal University, China. All participants received written informed consent in accordance with the Declaration of Helsinki. Four participants (1 male and 3 FMs) were deleted due to bad electroencephalogram data such as too much eye movements, blinks, muscle activities, unusual skin potentials, and clearly drifting waveforms.
Stimuli and Procedure
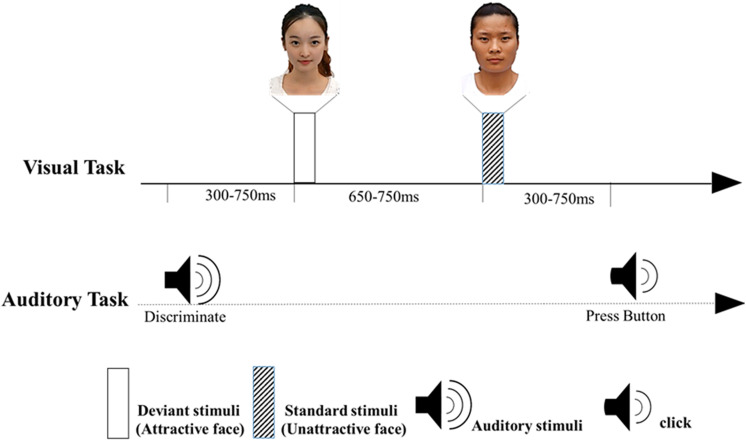
Figure 1 illustrates the stimuli and procedure. The presentation screen was a 17-in. computer monitor (1,024 × 768 resolution at 100 Hz refresh rate). The E-Prime 2.0 software (Psychological Software Tools, Inc., Pittsburgh, PA) was utilized to present the stimuli and record behavioral responses during the task.
Figure 1.
The paradigm “cross-modal and delayed response” shows part of trials. The visual images were either standard or deviant stimuli. (Between the tone and the click, 0 [no image], 1 [1 standard image or 1 deviant image], or 2 [2 standard images, 2 deviant images or 1 standard image, and 1 deviant image] face images were presented successively in pseudorandom order. This figure shows one way of presenting these images. The two participants had permitted to present their faces in Figure 1.)
A cross-modal delayed response paradigm (Wei et al., 2002) was used. Participants were instructed to focus their attention on the auditory stimuli delivered binaurally through earphones and ignored the visual stimuli. This design guaranteed that the participants’ attention was focused on the auditory task and that the perception of the attractive facial images presented in the oddball paradigm was unattended. There were five events in sequence, including two equiprobable target auditory stimuli (1,000 and 1,500 Hz), the standard and deviant stimuli in the visual modality, and an auditory response imperative signal. Every auditory stimulus was presented in each trial followed by 0–2 visual stimuli preceding a faint click (2 ms, 18 dB SPL-the sound unit) after the variable intervals of 300–2,250 ms (stimulus onset asynchrony [SOA]), which served as the response imperative signal. At the click, half of the participants were asked to quickly judge whether the tone was low or high in pitch by pressing either the horizontally adjacent “C” or “X” key on the keyboard using their left index or middle finger, and the other half responded with their right middle or index finger. To avoid motor activity artifacts, the participants did not respond before the click was presented. Between the tone and the click, 0 (no image), 1 (1standard image or 1 deviant image), or 2 (2 standard images, 2 deviant images or 1 standard image and 1 deviant image) face images were presented successively in pseudorandom order. The deviant images are the attractive facial images, and the standard images are the unattractive facial images. The images were always presented on the monitor approximately 90 cm from the participant for 100 ms with a visual angle of 12° × 10° at the center of monitor. The presentation order of the standard and deviant stimuli was pseudorandom. Whenever the images were presented, the SOA after the adjacent tone or the standard stimuli varied from 300 to 750 ms in pseudorandom order, and the SOA after the deviant stimuli varied from 650 to 750 ms, in order to guarantee the deviant-related components, which were obtained by subtracting ERPs of the standards from those of the deviants, were less likely to be contaminated by target effects than in the usual oddball paradigm (Wei et al., 2002). The intertrial interval randomly ranged between 1,000 and 1,500 ms. A total of 48 practice trials preceded the test trials. There were 320 test trials presented in two blocks (160 trials each). A total of 80 pictures were presented for fourth times. The images with unattractive face were repeated for 256 times (80%, standard stimulus), and the images with attractive face were respectively repeated for 64 times (20%, deviant stimuli). After stimuli runs, participants were instructed to rate the visual stimuli on a 5-point scale for attractiveness ranging from 1 (extremely unattractiveness) to 5 (extremely attractiveness).
Tone duration was 30 ms (including 5 ms rise/fall time), and the intensity was 60 dB SPL. In order to enhance the ecological validity, the images were color photographs from the original image pool. There were two kinds of facial images with no facial expression (so were the two kinds of facial images in Study 2). The male facial images were judged by female participants, and the female facial images were judged by male participants. The photographed students had previously consented to allow their pictures to be used in the studies of attractiveness. All pictures were taken under the same lighting conditions, distances, backgrounds, and framing (including hair and the top of the shoulders; Jung, Ruthruff, Tybur, Gaspelin, & Miller, 2012). Initially, the male original image gallery included 108 male face images of neutral expression. Sixty-one third-party female undergraduate students (Mage = 22.26, SD = 0.81) rated these faces on a 5-point scale ranging from 1 (extremely unattractiveness) to 5 (extremely attractiveness). None of these raters took part in ERP experiments of the study. So did to the female facial image gallery. Additionally, nine images were selected for preexperimental practice to orient the participants to the response screen. In order to match the percentage of trials, 80 male face images were selected for the current experiment, which can be categorized into attractive male face stimuli (mean rating = 4.92, SD = 0.26, n =16), unattractive male face stimuli (mean rating = 1.11, SD = 0.21, n = 64) images based on their mean ratings in the pilot study. Similar to the female face original images, 60 third-party male undergraduate students (Mage = 21.96, SD = 1.98) rated and picked 80 female face images of neutral expression, which can be categorized into attractive female face stimuli (mean rating = 4.89, SD = 0.39, n =16), unattractive female face stimuli (mean rating = 1.07, SD = 0.31, n = 64) images based on their mean ratings in the pilot study.
EEG recording and analysis
EEG was continuously recorded (band pass, 0.05–100 Hz; sampling rate, 500 Hz) using the Neuroscan SynAmp 2 Amplifier. An electrode cap with 64 Ag/AgCl electrodes was mounted according to the extended international 10-20 system, and the reference electrode was placed on the tip of the nose. Vertical Electrooculogram (VEOG) and Horizontal Electrooculogram (HEOG) were recorded using two pairs of electrodes: One was placed above and below the right eye, and another 10 mm from the lateral canthi. Electrode impedance was maintained below 5 kΩ to reduce the recorded artifacts throughout the experiment. During the task, electrophysiological signals and the presentation of stimuli were recorded continuously at a rate of 2,000 Hz. The EEG was re-referenced to the common average potential and was filtered off-line with a zero phase shift (bandwidth: 0.1–30 Hz, slope: 24 dB/octave).
The EEG was segmented in epochs of 700 ms, time-locked to picture onset, and included a 100-ms prestimulus baseline. To match the number of deviants, only 64 standard (unattractive facial images) images were averaged. The selection was pseudorandom so that only the standard images followed by an SOA of at least 650 ms were chosen. Electrooculogram (EOG) artifacts were corrected using the method proposed by Semlitsch et al. (Semlitsch, Anderer, Schuster, & Presslich, 1986). The trials contaminated by amplifier clipping, with bursts of electromyographic activity or peak-to-peak deflection exceeding ± 100 μV was excluded from averaging. The EEG segments were averaged separately for unattractive (averaged number = 220.6) and attractive (averaged number = 54.7) facial images.
The vMMN was calculated by subtracting the ERPs elicited by the standard stimuli (unattractive face) from those of the deviant stimuli (attractive face). Statistical analysis was based on within-subject factorial models in which the amplitudes (relative to the prestimulus baseline) of original ERP components (temporo-occipital N170 and P2 components) and subtraction-derived vMMN were dependent variables. The measurement windows were determined by visual inspection of grand-average waveforms 110–210 ms and 180–300 ms for the peak amplitudes and latency of N170 and P2, respectively, and three 140-ms time windows for vMMN (100–240 ms, 240–380 ms, and 380–520 ms). The vMMN is described as a negativity measured at the posterior scalp distribution (Stefanics et al., 2012). A set of studies provided convincing evidence for the existence of the vMMN right hemisphere advantage (Stefanics, Kimura, & Czigler, 2011; Zhao & Li, 2006; Kimura, Schröger, & Czigler, 2011). So there were six bilateral symmetry electrode sites (left: P7, PO7, and O1; right: P8, PO8, and O2) being selected in the current study. For N170 and P2, peak amplitudes and latency were assessed via repeated-measures analysis of variance (ANOVA) using the factors for Group (FM/M) × Attractiveness (high and low) × Hemisphere (left and right) × Site (P7/P8, PO7/PO8, and O1/O2). For the vMMN, peak amplitudes and latency were assessed via repeated-measures ANOVA using the factors for Group (FM/M) × Hemisphere (left and right) × Site (P7/P8, PO7/PO8, and O1/O2). The degrees of freedom were corrected using the Greenhouse-Geisser epsilon. Post hoc testing of significant main effects was conducted using a Bonferroni correction, and significant interactions were further analyzed using a simple-effects model. Partial eta squared ( ) was reported to demonstrate the effect size of the ANOVA tests. One-tailed t tests determined whether the amplitudes of vMMN were significantly different from zero.
Results
Behavioral Data
In order to know the effect of attention, the accuracy of auditory stimuli was evaluated. The behavioral performances (i.e., detection of 95.7% and 92.4% of 1,000- and 1,500-Hz auditory stimuli, respectively) demonstrated that the participants’ attention was focused on the auditory modality. Given the large number of participants in this study, the power of the vMMN might be facilitated. The facial attractiveness ratings collected at the end of the session using the 5-point attractiveness scale generally mirrored the average ratings from the pilot study. The average rating for the attractive face was 4.72 (SD = 0.32) compared to the pilot study (mean = 4.89, SD = 0.39). The average rating for the unattractive face was 1.42 (SD = 0.37) compared to the pilot study (mean = 1.07, SD = 0.31).
ERPs Data
N170 and P2 components
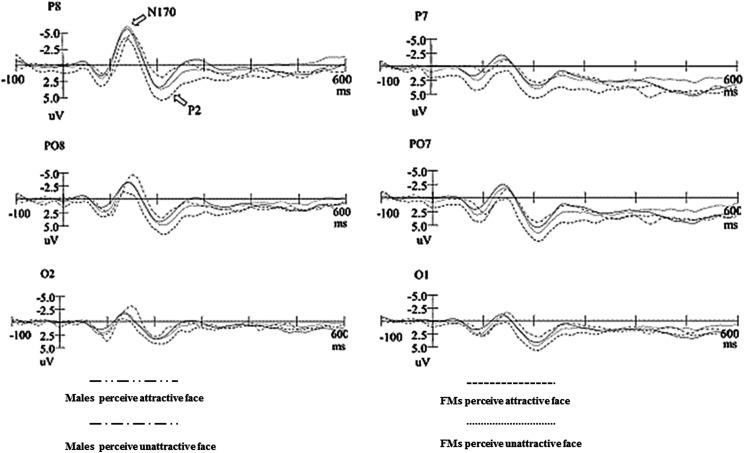
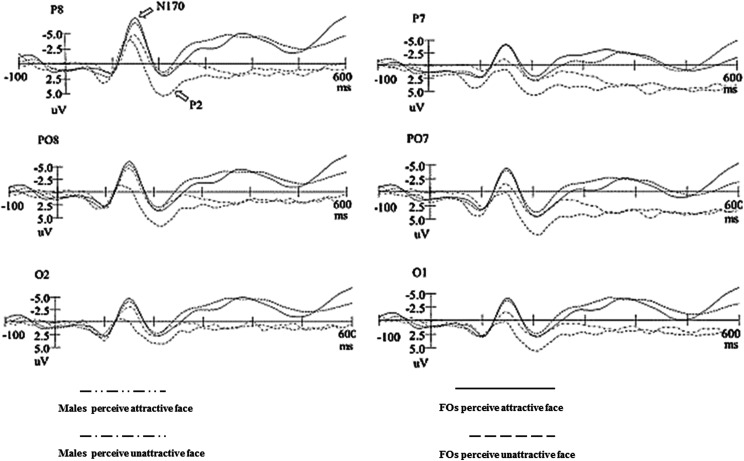
As shown in Figure 2, grand-averaged ERPs were elicited by unattractive facial images (standard stimuli) and attractive facial images (deviant stimuli). All stimuli elicited N170 and P2 components at the posterior scalp. The attractive face elicited larger N170 and smaller P2 than the unattractive face. Moreover, FMs had more negative N170 potentials than males (Figure 2).
Figure 2.
Grand-average event-related potentials in response to attractive face (deviant stimuli) and unattractive face (standard stimuli) stimuli for males and females in menstrual period.
First, for the amplitude, the main effect of group for the amplitude of N170 was significant, F(1, 58) = 36.139, p < .001, = .384, reflecting that FMs had larger N170 (−4.220 μV) than males (−1.732 μV). But the main effect of group for the amplitude of P2 was not significant. The main effects of attractiveness for the amplitude of N170 and P2 were also significant, F(1, 58) = 22.108, p < .001, = .276; F(1, 58) = 10.543, p = .002, = .163, respectively, reflecting that attractive faces elicited larger N170 (−3.521 μV) and smaller P2 (3.760 μV) than unattractive faces (N170: −2.430 μV, P2: 5.990 μV). The main effects of hemisphere for the amplitude of N170 and P2 were also significant, F(1, 58) = 23.037, p < .001, = .284; F(1, 58) = 5.214, p = .026, = .088, respectively, reflecting that the right hemisphere had larger N170 (−3.715 μV) and smaller P2 (4.434 μV) than the left hemisphere. The main effects of site for the amplitude of N170 and P2 were significant, F(1.577, 91.489) = 23.037, p < .001, = .284; F(1.521, 92.159) = 9.820, p = .001, = .154, respectively, with the biggest amplitude at P7/P8 site for N170 (−6.081 μV) and at PO7/PO8 site for P2 (5.519 μV). Additionally, a significant interactions of Group × Attractiveness for the amplitude of N170 and P2 were found, F(1, 58) = 21.030, p < .01, = .266; F(1, 58) = 4.933, p = .031, = .084, respectively. Simple effect analysis reflected that attractive faces elicited larger N170 and smaller P2 than unattractive faces for male participants (N170: −2.809 μV vs. −.655 μV; P2: 3.392 μV vs. 7.149 μV; Figure 2). The Attractiveness × Site interaction on P2 was also significant, F(1.363, 83.587) = 5.362, p = .015, = .090, with the biggest amplitude at PO7/PO8 site (7.000 μV) for unattractive faces. There were no other significant interactions for N170 and P2.
In addition, the differences in the latency of N170 among groups were significant, F(1, 58) = 6.116, p = .016, = .095, reflecting the latency of N170 in FMs was shorter (138.775 ms) than males (144.256 ms). The difference of N170 latency between attractive and unattractive faces was also significant, F(1, 58) = 4.920, p = .030, = .078, reflecting the latency of N170 was shorter for unattractive faces (139.100 ms) relative to attractive faces (143.931 ms). For the latencies of P2, neither main effects nor interactions were significant.
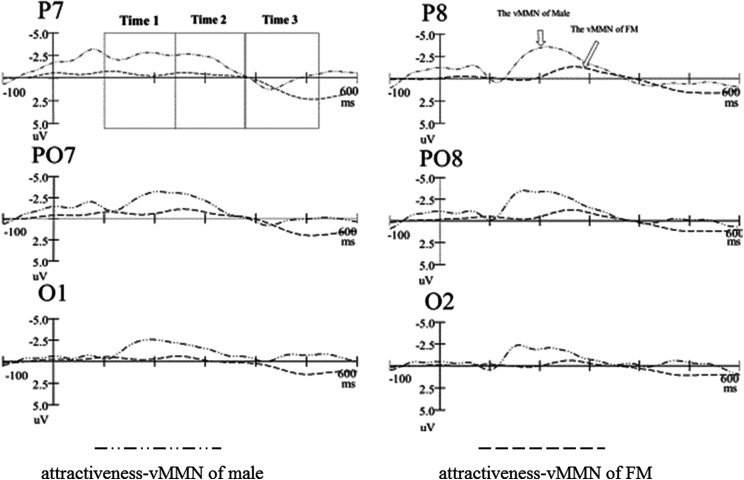
Attractiveness vMMN
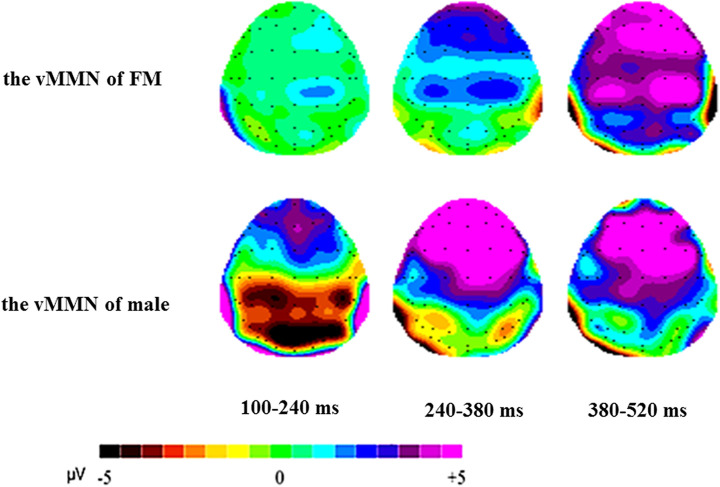
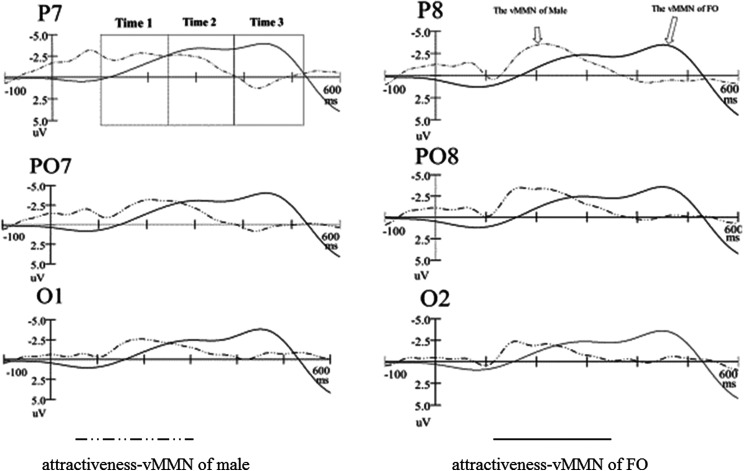
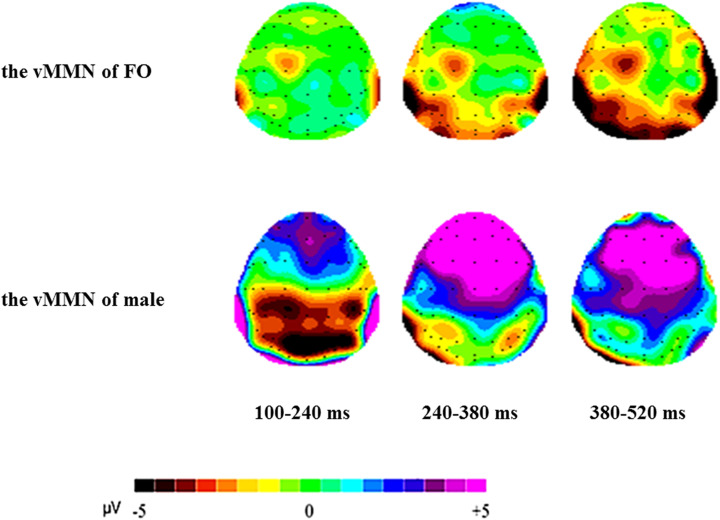
Figure 3 presented the grand-average attractiveness vMMN for FMs and males, peaked at 270 and 212 ms poststimuli, respectively. Clearly, the males had most negative attractiveness vMMN (−4.912 μV at 212 ms) in the early stage. Figure 4 showed the 2D scalp topographic distribution of vMMN in three different intervals (100–240 ms, 240–380 ms, and 380–520 ms). It is conspicuous that vMMN was distributed at posterior areas: parietal-occipital (e.g., PO8 and O2) areas and parietal-temporal (e.g., P8) areas.
Figure 3.
The grand-average attractiveness visual mismatch negativity of males and females in menstrual period.
Figure 4.
The 2D scalp topographic distributions of the visual mismatch negativity of males and females in menstrual period.
The statistical reliability of the above pattern was tested by a three-factor ANOVA of the attractiveness vMMN mean amplitudes (100–240 ms, 240–380 ms, and 380–520 ms, respectively), with group (FMs and males) as between-subject factor, hemisphere (left and right) and site (O1/O2, PO7/PO8, and P7/P8) as within-subject factors. In the 100- to 240-ms time window, the main effect of group for amplitude of vMMN was statistically significant, F(1, 59) = 236.577, p < .001, = .809, reflecting that the attractiveness vMMN in male was more negative (−4.916 μV) than FMs (−1.386 μV). The main effects of site were statistically significant, F(1.960, 109.746) = 3.764, p = .027, = .063, showing that the amplitude of vMMN was largest (−3.098 μV) at P7/P8 site. The statistical significance was not reached in all of the interactions.
In the 240- to 380-ms time window, the main effect of group for the amplitude of vMMN was statistically significant, F(1, 59) = 102.983, p < .001, = .648, reflecting that the attractiveness vMMN for males (−2.507 μV) was more negative than FMs (−.923 μV). The main effect of site was statistically significant, F(1.941, 108.668) = 10.345, p < .001, = .156, showing the amplitude of vMMN was largest (−2.128 μV) at P7/P8 site. Statistical significance was not reached in the main effect of hemisphere and all of the interactions.
In the 380- to 520-ms time window, the main effect of group for the amplitude of vMMN was not statistically significant between males (−.390 μV) and FMs (−.196 μV). Statistical significance was not reached in the main effect of hemisphere, site, and all of the interactions.
Additionally, the peak amplitude of males was much larger than FMs, t(58) = 15.381, p < .001, and the latency of males was longer than FMs, t(58) = 9.962, p < .001.
Discussion
In Study 1, the attractiveness vMMN was recorded. As expected, the vMMN was obtained on posterior scalp distribution (posterior-occipital and occipital areas), indicating that the attractive faces can be perceived automatically. More importantly, males showed a stronger vMMN activation (male: −4.916 μV at 270 ms) than FMs (FMs: −1.386 μV at 212 ms), demonstrating that attractive opposite-sex faces can be processed more automatically by males than by FMs. This suggests that attractive faces produced an attentional superiority effect for male.
Study 2
In Study 2, we examined the automatic processing of highly attractive opposite-sex faces in FOs and males.
Method
Participants
A total of 64 healthy right-handed (measured by the Reitan Test) undergraduate students (19–23 years; 32 FOs and 32 males were awarded extra course credit in exchange for their participations). The female participants were asked how many days have passed since onset of their last period of menses. When the ovulatory periods of female participants are approaching (14 days after menses start), they are informed to use luteinizing hormone test trip for ovulatory test. Only participants in the ovulatory phase were invited for experiments (Beall & Tracy, 2013; Gonzales & Ferrer, 2015). Otherwise they were asked to wait till the ovulatory phase came. The participants all had normal or corrected-to-normal visual acuity and normal hearing. None of them reported any history of neurological or mental diseases. And their sexual orientations are all heterosexual. This study was carried out in accordance with the recommendations of Ethical Principles of Psychologists and Code of Conduct, The American Psychological Association. The protocol was approved by the Research Ethics Committee of Shandong Normal University, China. All participants received written informed consent in accordance with the Declaration of Helsinki. Four participants (two males and two FOs) were deleted due to bad EEG data, due to too much eye movements, blinks, muscle activates, skin potentials, and clearly drifting waveforms.
The Stimuli Used in Study 2 and the Procedure Were the Same as Study 1
EEG recording and analysis
EEG was continuously recorded (band pass, 0.05–100 Hz; sampling rate, 500 Hz) using the Neuroscan SynAmp 2 Amplifier. An electrode cap with 64 Ag/AgCl electrodes was mounted according to the extended international 10-20 system, and the reference electrode was placed on the tip of the nose. VEOG and HEOG were recorded using two pairs of electrodes: One was placed above and below the right eye, and another 10 mm from the lateral canthi. Electrode impedance was maintained below 5 kΩ to reduce the recorded artifacts throughout the experiment. During the task, electrophysiological signals and the presentation of stimuli were recorded continuously at the rate of 2,000 Hz.
The EEG was segmented in epochs of 700 ms, time-locked to picture onset, and included a 100-ms prestimulus baseline. In the current study, more standard stimuli were presented than deviants (256 vs. 64). According to Wei et al. (2002), for the short SOA between the stimuli might induce the overlapping of the ERPs, only 64 standard (neutral) images with SOA of 650 ms or longer were selected for averaging to equalize the number of averaging deviants (Wei et al., 2002; Zhao & Li, 2006). EOG artifacts were corrected using the method proposed by Semlitsch et al. (1986). The trials contaminated by amplifier clipping with bursts of electromyographic activity or peak-to-peak deflection exceeding ± 100 μV were excluded from averaging. The EEG segments were averaged separately for unattractive (averaged number = 231.5) and attractiveness (averaged number = 55.1) facial images. The EEG was re-referenced to the common average potential and was filtered off-line with a zero phase shift (bandwidth: 0.1–30 Hz, slope: 24 dB/octave).
The vMMN was calculated by subtracting the ERPs elicited by the standard stimuli (unattractive face) from those of the deviant stimuli (attractive face). Statistical analysis was based on within-subject factorial models in which the amplitudes (relative to the prestimulus baseline) of original ERP components (temporo-occipital N170 and P2 components) and subtraction-derived vMMN were dependent variables. The measurement windows were determined by visual inspection of grand-average waveforms 110–210 ms and 180–300 ms for the peak amplitudes and latency of N170 and P2, respectively, and three 140-ms time windows for vMMN (100–240 ms, 240–380 ms, and 380–520 ms). There were six electrode sites (left: P7, PO7, and O1; right: P8, PO8, and O2) selected. For N170 and P2, peak amplitudes and latency were assessed via repeated-measures ANOVA using the factors for Group (FO/M) × Attractiveness (high and low) × Hemisphere (left and right) × Site (P7/P8, PO7/PO8, and O1/O2). For the vMMN, peak amplitudes and latency were assessed via repeated-measures ANOVA using the factors for Group (FO/M) × Hemisphere (left and right) × Site (P7/P8, PO7/PO8, and O1/O2). The degrees of freedom were corrected using the Greenhouse-Geisser epsilon. Post hoc testing of significant main effects was conducted using a Bonferroni correction, and significant interactions were further analyzed using a simple-effects model. Partial eta squared ( ) was reported to demonstrate the effect size of the ANOVA tests. One-tailed t tests were conducted to determine whether the vMMN amplitudes were significantly different from zero.
Results
Behavioral Data
In order to examine the effect of attention, the accuracy of auditory stimuli was evaluated. The behavioral performances (i.e., detection of 95.1% and 91.3% of 1,000 and 1,500 Hz auditory stimuli, respectively) demonstrated that the participants’ attention was focused on the auditory modality. Given the large number of participants in this study, the power of the vMMN might be facilitated. The facial attractiveness ratings collected at the end of the session using the 5-point attractiveness scale generally mirrored the average ratings from the pilot study. The average rating for the attractive face was 4.79 (SD = 0.31) compared to the pilot study (mean = 4.89, SD = 0.39). The average rating for the unattractive face was 1.22 (SD = 0.36) compared to the pilot study (mean = 1.07, SD = 0.31).
ERPs Data
N170 and P2 components
As shown in Figure 5, grand-averaged ERPs were elicited by unattractive facial images (standard stimuli) and attractive facial images (deviant stimuli). All stimuli elicited N170 and P2 components at the posterior scalp. The attractive face elicited larger N170 and smaller P2 than the unattractive face. Moreover, FOs had more negative N170 potentials than males (Figure 5).
Figure 5.
Grand-average event-related potentials in response to attractive face (deviant stimuli) and unattractive face (standard stimuli) stimuli for males and females in ovulatory period.
First, for the amplitude, the main effect of group for amplitude of N170 was significant, F(1, 58) = 76.962, p < .001, = 0.570, reflecting that FOs had the larger amplitude of N170 (−6.081 μV) than males (−1.732 μV). But the main effect of group for amplitude of P2 was not significant. The main effects of attractiveness for amplitude of N170 and P2 were also significant, F(1, 58) =11.934, p = .001, = .171; F(1, 58) = 9.120, p = .004, = .136, respectively, reflecting that attractive faces elicited larger amplitude of N170 (−4.455 μV) and smaller amplitude of P2 (4.823 μV) than unattractive faces (N170: −3.358 μV, P2: 6.229 μV). The main effects of hemisphere for amplitude of N170 and P2 were also significant, F(1, 58) = 46.740, p < .001, = .446; F(1, 58) = 4.870, p = .031, = .077, respectively, reflecting that the right hemisphere had larger amplitude of N170 (−4.880 μV) and smaller amplitude of P2 (5.217 μV) than the left hemisphere. The main effects of site for amplitude of N170 and P2 were significant, F(1.816, 105.344) = 12.914, p < .001, = .182; F(1.655, 96.209) = 23.380, p < .001, = .287, respectively, with the biggest amplitude at PO7/PO8 site for N170 (−6.758 μV) and at PO7/PO8 site for P2 (6.330 μV). Additionally, a significant interactions of Group × Attractiveness of N170 and P2 were found, F(1, 58) =11.047, p = .02, = .160; F(1, 58) = 25.436, p < .01, = .305, respectively. Simple effect analysis reflected that attractive faces elicited larger amplitude of N170 and smaller amplitude of P2 than unattractive faces in male participants (N170: −2.809 μV vs. −0.655 μV; P2: 3.392 μV vs. 7.149 μV; Figure 5). The Attractiveness × Site interaction of P2 was also significant, F(1.409, 116.971) = 9.583, p < .01, = .142, with the biggest amplitude at PO7/PO8 site (7.314 μV) for unattractive faces. There were no other significant interactions for N170 and P2.
In addition, the differences in N170 latency between two groups were not significant, reflecting the latency of N170 in FOs was similar as males. The difference for latency of N170 between attractive and unattractive faces was also significant, F(1,58) = 6.254, p = .015, = .104, reflecting the latency of N170 was shorter for unattractive faces (140.610 ms) relative to attractive faces (148.902 ms). For the latencies of P2, neither main effects nor interactions were significant.
Attractiveness vMMN
Figure 6 presents the grand-average attractiveness vMMN for FOs and males, peaked at 450 ms and 212 ms poststimuli, respectively. Clearly, the males had most negative attractiveness vMMN (−4.912 μV at 270 ms) in the early stage, and the FMs had most negative attractiveness vMMN (−4.616 μV at 450 ms) in the late stage. Figure 7 shows the 2D scalp topographic distribution of vMMN in three different intervals (100–240 ms, 240–380 ms, and 380–520 ms). It is conspicuous that vMMN was distributed at posterior areas: parietal-occipital (e.g., PO8 and O2) areas and parietal-temporal (e.g., P8) areas.
Figure 6.
The grand-average attractiveness visual mismatch negativity for males and females in ovulatory period.
Figure 7.
The 2D scalp topographic distributions of the attractiveness visual mismatch negativity of males and females in ovulatory period.
The statistical reliability of the above pattern was tested by a three-factor ANOVA of the attractiveness vMMN mean amplitudes (100–240 ms, 240–380 ms, and 380–520 ms, respectively), with group (FOs and males) as between-subject factor, hemisphere (left and right) and site (O1/O2, PO7/PO8 and P7/P8) as within-subject factors. In the 100- to 240-ms time window, the main effect of group for amplitude of vMMN was statistically significant, F(1, 59) = 71.182, p < .001, = .555, reflecting that the amplitude of attractiveness vMMN in male was more negative (−4.916 μV) than FOs (−2.313μV). The main effects of hemisphere and site were statistically significant, F (1, 59) = 5.630, p = .021, = .090; F(1.972, 112.807) = 5.319, p = .006, = .085, respectively, showing that the amplitude of vMMN was largest (−3.098 μV) at P7 site. The statistical significance was not reached in all of the interactions.
In the 240- to 380-ms time window, the main effect of group for amplitude of vMMN was not statistically significant (males [−2.409 μV] and FOs [−2.335 μV]). The main effect of site was statistically significant, F(1.961, 111.760) = 6.936, p = .002, = .108, showing the vMMN amplitude was the largest (−2.128 μV) at P7/P8 site. Statistical significance was not reached in the main effect of hemisphere and all of the interactions.
In the 380- to 520-ms time window, the main effect of group for amplitude of vMMN was also statistically significant, F(1, 59) = 554.184, p < .001, = .907, showing the attractiveness vMMN for FOs was more strongly negative (−4.613 μV) than males (−0.390 μV). Statistical significance was not reached in the main effect of hemisphere, site, and all of the interactions.
Additionally, the peak amplitude of FOs was similar as males, t(58) = 1.109, p = .272, but the latency of FOs was much longer than males, t(58) = 37.920, p < .001.
Discussion
In Study 2, the attractiveness vMMN was recorded. As expected, the vMMN was obtained on posterior scalp distribution (posterior-occipital and occipital areas), indicating that attractive faces can be perceived automatically. More importantly, FOs (−4.613 μV at 450 ms) and males (male: −4.916 μV at 270 ms) have strong vMMN activation in similar magnitude but in different time window. This demonstrated that there are same degree and different priorities of the automatic perception between the two groups (the latency of vMMN of FOs is longer than males).
Comparison of Study 1 and Study 2
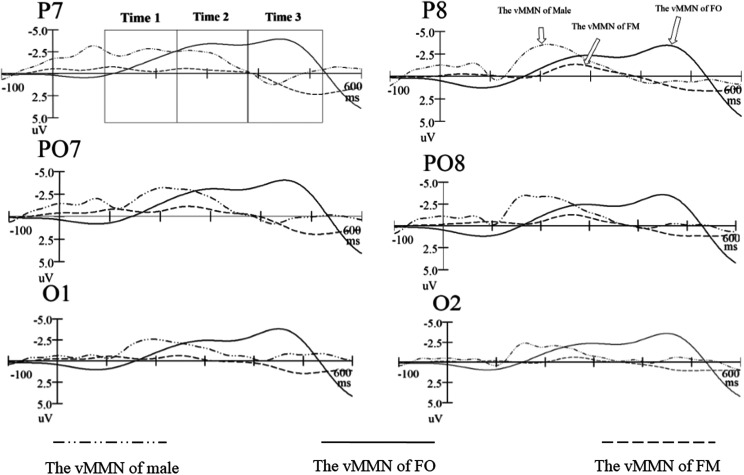
Figure 8 presents the grand-average attractiveness vMMN for FOs, FMs, and males, peaked at 450, 270, and 212 ms poststimuli, respectively. Clearly, males had the most negative attractiveness vMMN (−4.916 μV at 270 ms) in the early stage, and FMs had the most negative attractiveness vMMN (−4.613 μV at 450 ms) in the late stage.
Figure 8.
The grand-average attractiveness visual mismatch negativity for males, females in ovulatory period, and females in menstrual period.
The statistical reliability of the above pattern was tested by a three-factor ANOVA of the attractiveness vMMN mean amplitudes (100–240 ms, 240–380 ms, and 380–520 ms, respectively), with group (FOs, FMs and males) as between-subject factor, hemisphere (left and right) and site (O1/O2, PO7/PO8, and P7/P8) as within-subject factors. In the 100- to 240-ms time window, the main effect of group for amplitude of vMMN was statistically significant, F(2, 85) = 98.669, p < .001, = .699, reflecting that the amplitude of attractiveness vMMN in male was most negative (−4.916 μV), the amplitude of attractiveness vMMN in FOs was more negative (−2.313μV) than FMs (−1.386 μV). The main effects of site were statistically significant, F(1, 85) = 6.909, p = .010, = .075; F(1.972, 167.588) = 4.726, p = .010, = .053, respectively, showing that the amplitude of vMMN was largest (−3.098 μV) at P7 site. The statistical significance was not reached in all of the interactions.
In the 240- to 380-ms time window, the main effect of group for amplitude of vMMN was statistically significant, F(2, 85) = 47.885, p < .001, = .574, reflecting that the amplitude of attractiveness vMMN in males (−2.595 μV) and FOs (−2.236 μV) was more negative than FMs (−.923 μV). The main effect of site was statistically significant, F(1.971, 167.575) = 11. 563, p < .001, = .120, showing that the amplitude of vMMN was the largest (−2.128 μV) at P7/P8 site. Statistical significance was not reached in the main effect of hemisphere and all of the interactions.
In the 380- to 520-ms time window, the main effect of group for amplitude of vMMN was also statistically significant, F(2, 85) = 501.664, p < .001, = 0.922, showing the amplitude of attractiveness vMMN in FOs was most strongly negative (−4.613 μV), and the amplitude of attractiveness vMMN in males was more negative (−0.390 μV) than FMs (−0.196 μV). Statistical significance was not reached in the main effect of hemisphere, site, and all of the interactions.
In conclusion, the peak amplitude of attractiveness vMMN in FOs (−4.613 μV) and males (−4.916 μV) was larger than FMs (−1.386 μV), which were statistically significant, F(2, 85) = 142.710, p < .001, = .770. But the difference between the peak amplitude of attractiveness vMMN in FOs (−4.613 μV) and males (−4.916 μV) was not statistically significant (p = .192). For the latency of attractiveness vMMN, FOs was the longest among the three kinds of groups, F(2, 85) = 1,527.894, p < .001, = .944. This demonstrating that there are different priorities of the automatic perception among the three groups (male is earliest, FM is middle, FO is latest in the latency of vMMN), and attractive faces produced an attentional superiority effect that has evolutionary advantage.
General Discussion
Although the detection of attractive face has been investigated, the brain mechanism of attractive facial perception in females with different physiological cycles and males is unclear. The central aim of this study was to investigate the mechanism of attractiveness perception under unattended condition with a modified cross-modal delayed response paradigm. In the two experiments, the attractiveness vMMN could be obtained for all groups but occurred in different time courses for different groups.
Overall speaking, the pronounced attractiveness vMMN on posterior scalp distribution (posterior-occipital and occipital areas) demonstrated that all participants can visually detect attractive faces automatically in the context of unattractive face stimuli. From an evolutionary perspective, perception of attractiveness serves as the adaptation result for seeking mate with good quality. As we know, facial attractiveness is a reliable cue of the owner’s biological quality and mate value because attractive people have better parasite resistance, physical and reproductive fitness, longevity, less mutational load, higher intelligence, and better mental health. Thus, people with the capacity of automatically perceive high-attractive opposite-sex face have good chances to get erotic access to an opposite sex and thereby to increase their reproductive success. Based on the adaptation-oriented explanation, it posits that this automatic perception of attractive faces is an evolutionary adaptation and the result of natural selection.
Specially, we found that the amplitude of vMMN in males was much larger than FMs in Study 1 but similar as FOs in Study 2. Previous studies found that males in short bond prefer females with fertility characteristics, while males in the long bond prefer females with high reproductive value (Buss & Barnes, 2015; Buss & Schmitt, 1993; Hooff et al., 2011; Young, Critelli, & Keith, 2005). Both fertility and reproductive value can be reflected in females’ faces. Taken together, the results indicated that attractiveness is of the most importance for males in many mate choice criteria, no matter in long bond and the short bond. Good genes theory holds that attractive face is considered the symbol of healthy genes, sound immunity, and reproductive advantage, hinting more successful reproduction (Rhodes, 2006). Therefore, males may have the capacity of automatic attractiveness perception and have larger ERP effects produced by female face. Otherwise, the face that, the amplitude of N170 in males for attractive female faces was larger than unattractive female faces, can also support this, which is consistent with other studies (Lu, Wang, Wang, Wang, & Qin, 2014; Marzi & Viggiano, 2010; Zhang & Deng, 2012).
In consistent with other studies, we found that males and females showed differences in the preferences for highly attractive opposite-sex faces: FOs and males were similar but FMs are different (Aharon et al., 2001; Choi et al., 2015; Cloutier et al., 2008; Iaria et al., 2008; Ishai, 2007; Kranz & Ishai, 2006; Penton-Voak et al., 2004; Senior, 2003). More importantly, the amplitude of vMMN in FOs was as big as the males, but much larger than the FMs. These findings indicated the automatic perception of high-attractive opposite-sex faces in FOs may be underpinned by the breeding motivation. It can be explained in two ways. First, as FOs can realize their own genetic inheritance and are driven by a strong motivation for breeding, they have more intense incentives to pursue and attract sexual partners in order to have higher quality offspring. Under the motivation of breeding, ovulating females will conduct more frequent sexual behaviors, and even seek short-term sexual partners, so that they tend to invest more psychological resources in case of the opposite sex with high attractiveness (Gueguen, 2009; Röder, Brewer, & Fink, 2009). Therefore, FOs prefer highly attractive male faces (which signal good genes), which is similar to males (Gangestad, Thornhill, & Garver-Apgar, 2005; Johnston, Hagel, Franklin, Fink, & Grammer, 2001; Little, Jones, Burt, et al., 2007; Little, Jones, Pentonvoak, Burt, & Perrett, 2002). That is why the amplitude of vMMN in FOs was similar as the males. Second, the amplitude of vMMN in FOs is much larger than FMs, reflecting that FOs may be more interested in high-attractive male faces than FMs. The possible explanation is that the opposite-sex facial preferences in females are related to the menstrual cycle and based on the breeding motivation. FOs are more likely to have higher levels of sexual arousal than FMs. So that they tend to be more sensitive to male’s facial feature (Gangestad & Thornhill, 1998; Little, Jones, Burt, et al., 2007), even considered the masculine and symmetrical males were more attractive (Little, Jones, & Debruine, 2008; Penton-Voak et al., 2003; Welling et al., 2007). By comparison, the amplitude of vMMN in FMs is the smallest of all groups, so the vMMN of attractive opposite-sex faces in FMs is less automated. This indicated that the reproductive motivation is weak in this stage, and the perception of the attractive opposite-sex faces has a small effect on mate selection. Therefore, from the perspective of evolutionary psychology, females in the high fertile period (the ovulatory period) would pay more attention to the high genetic quality represented by the attractive opposite-sex face, in contrast, females in the low fertile period (the menstrual period) are interested in characteristics indicating parental investment.
Another interesting phenomenon was that the vMMN of FOs appears latest compared with the males and FMs in both the experiments, which was related to FOs’ other proliferation motivation—the motivation to avoid being tainted by genes, a danger that is most significant only in ovulation period. In this period, females are most likely to have their genes contaminated in the whole reproductive process (Navarrete, Fessler, Fleischman, & Geyer, 2009). And they may also be confronted with huge personal costs in the case of forced pregnancies (Garverapgar, Gangestad, & Simpson, 2007; Thornhill & Palmer, 2000). Under this motivation, they would prudently overestimate the opposite sex as a sexual aggressor regardless of wrong judgment and even dodge out-group to avoid the risk of being sexually assaulted (Mcdonald, Asher, Kerr, & Navarrete, 2011; Navarrete et al., 2009). This motivation can also be supported by the face-sensitive component N170, which showed differences between FOs and FMs about perceiving the attractive and unattractive opposite-sex faces. The amplitude of N170 in FOs was much higher than FMs, but there were no difference between perceiving the attractive and unattractive male faces in FOs and FMs, which have not been found in previous studies. This indicated that, in order to distinguish whether the opposite-sex face’s owner is an out-group or carrying a faulty gene, they allocate more attention resources, which were reflected in the largest amplitude of N170 and the longest latency of vMMN in FOs. In addition, through analyzing the latency of N170 and vMMN in FOs, we can see that the motivation to avoid the genetic stain takes priority over the breeding motivation. Hence, what females do first lies in ruling out the risk of gene pollution, on the basis of which they will further screen high-quality genes to breed their offspring. This is similar as some other behavior studies (Gangestad, Thornhill, & Garver-Apgar, 2010; Little, Jones, & Burriss, 2007).
In terms of neural mechanism, the attractiveness vMMN obtained in this study was distributed at posterior areas (parieto-occipital and parieto areas). These results are consistent with previous studies investigating the process of attractive facial information. Attractive faces will stimulate some cerebral regions related to reward and emotion, such as orbitofrontal cortex, amygdaloid nucleus, basal ganglia. (Ishai, 2007; Wilson & Daly, 2004; Winston et al., 2007). Some research made changes to experiment tasks with attractive and unattractive faces, but no matter how the change was, VTA would invariably be automatically activated by attractive faces (Chatterjee et al., 2009). Meanwhile, the attractiveness vMMN provided more convincing evidence about the automatic processing of the attractive faces. Meanwhile, the standardized low-resolution brain electromagnetic tomography method might help us to explore the cortical generators of attractiveness vMMN (AMMN) in future studies.
In addition, even though a lot of studies do not consistent with the conclusion, the female perception to male attractive faces differs with menstrual phase, drawn from this study (Harris, 2011, 2013; Jones, 2018; Muñoz-Reyes, Pita, Arjona, Sanchez-Pages, & Turiegano, 2014; Wood & Carden, 2014; Zietsch, Lee, Sherlock, & Jern, 2015). These controversies should be studied in future. However, this study has revealed in the time course of attractive facial perception, which may provide sounder evidence showing that females’ automatic perception of facial attractiveness varies across the menstrual cycle.
This study had some limitations. First, previous studies indicated that the traditional vMMN in the oddball sequence indeed confounds standard stimuli refractoriness reflected by the changes of early visual ERP components such as the temporo-occipital N1 component (Astikainen & Hietanen, 2009; Chang, Xu, Shi, Zhang, & Zhao, 2010; Stefanics, Csukly, Komlósi, Czobor, & Czigler, 2012; Susac, Ilmoniemi, Pihko, Ranken, & Supek, 2010; Susac, Ilmoniemi, Pihko, & Supek, 2004) and N170 component (Japee, Crocker, Carver, Pessoa, & Ungerleider, 2009; Vlamings, Goffaux, & Kemner, 2009; Wronka & Walentowska, 2011), due to its similar latency and scalp topography with vMMN (Luck, 2014). Astikainen, Cong, Ristaniemi, and Hietanen (2013) found two separate components for the emotional faces in both the oddball and equiprobable conditions by independent component analysis (Astikainen, Cong, Ristaniemi, & Hietanen, 2013). A component peaking at 130 ms poststimulus showed a difference in scalp topography between the oddball (bilateral) and the equiprobable (right-dominant) conditions. So the condition oddball (equiprobable deviant stimuli) paradigm (Kimura, Katayama, Ohira, & Schröger, 2009; Stefanics, Kremláček, & Czigler, 2014a) might be used to testify whether the attractive-vMMN is influenced by the rarity of deviant stimuli or the emotional information of attractive facial images in future studies. Second, FMs and FOs were compared in this study. The change of female hormone level is influenced by many factors, including age and physiological cycle, and the division of physiological cycle is relatively complicated. In the future studies, females in the follicular and luteal periods should also be studied in a comprehensive and detailed manner. Third, more accurate methods in measuring hormone level, such as blood tests, were not adopted in this study. In the future research, this shortcoming is expected to be overcome with improved experiment technology. Moreover, this study found that FMs were not interested in attractive opposite-sex faces, then which kinds of male faces might arouse their interest could be the future research direction.
In conclusion, the results of this study suggest that the attractive opposite-sex faces can be perceived automatically and the allocation of attention can be adaptively modulated by observers’ own physiological factor. For males, in order to gain high-quality mate, attractive female faces tend to trigger more intense and earlier ERP responses. For females at different stages of physiological cycle, their hormone level and reproductive ability change accordingly, so does their visual preference over male faces. Meanwhile, females also show the high and later automatic perception of attractive opposite-sex faces during ovulatory period. These findings can be explained by the fact that ovulating females have two different motivations: The motivation to avoid the genetic staining and the motivation to reproduce, with the former taking precedence over the latter. And based on the breeding motivation, FOs crave for good genes that they pass on to their offspring, as males do. The perception of high-attractive opposite-sex faces is commonly regarded as adaptations, which have evolved in the course of biological history. This feature not only has evolution significance but also sociological significance.
Footnotes
Authors’ Note: Shu Zhang conceived and designed the experiment and written the manuscript. Hailing Wang and Qingke Guo performed the analysis with constructive discussions. Shu Zhang, Hailing Wang and Qingke Guo revised the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by National Social Science Fund (15BSH083) of People’s Republic of China for QG, the National Science Foundation of People’s Republic of China (31700940) and project funded by China Postdoctoral Science Foundation (2017M622250) for HW, and the Human Cognition and behavior Development” Key Laboratory Project of Shandong Province.
ORCID iD: Shu Zhang  https://orcid.org/0000-0002-2548-502X
https://orcid.org/0000-0002-2548-502X
Qingke Guo  https://orcid.org/0000-0002-3472-3886
https://orcid.org/0000-0002-3472-3886
References
- Aharon I., Etcoff N., Ariely D., Chabris C. F., O’Connor E., Breiter H. C. (2001). Beautiful faces have variable reward value—fMRI and behavioral evidence. Neuron, 32, 537–551. [DOI] [PubMed] [Google Scholar]
- Astikainen P., Cong F., Ristaniemi T., Hietanen J. K. (2013). Event-related potentials to unattended changes in facial expressions: Detection of regularity violations or encoding of emotions? Frontiers in human neuroscience, 7, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astikainen P., Hietanen J. K. (2009). Event-related potentials to task-irrelevant changes in facial expressions. Behavioral and Brain Functions, 5, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astikainen P., Lillstrang E., Ruusuvirta T. (2008). Visual mismatch negativity for changes in orientation—A sensory memory-dependent response. European Journal of Neuroscience, 28, 2319–2324. [DOI] [PubMed] [Google Scholar]
- Beall A. T., Tracy J. L. (2013). Women are more likely to wear red or pink at peak fertility. Psychological Science, 24, 1837–1841. [DOI] [PubMed] [Google Scholar]
- Berti S. (2011). The attentional blink demonstrates automatic deviance processing in vision. Neuroreport, 22, 664–667. [DOI] [PubMed] [Google Scholar]
- Boothroyd L. G., Gray A. W., Headland T. N., Uehara R. T., Waynforth D., Burt D. M., Pound N. (2017). Male facial appearance and offspring mortality in two traditional societies. PLos One, 12, e0169181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstad P. M., Russell R. (2007). Beauty is in the “we” of the beholder: Greater agreement on facial attractiveness among close relations. Perception, 36, 1674–1681. [DOI] [PubMed] [Google Scholar]
- Buss D. M. (2005). The evolutionary psychology handbook. New York, NY: Wiley. [Google Scholar]
- Buss D. M. (2015). Evolutionary psychology: The new science of the mind. Hove, England: Psychology Press. [Google Scholar]
- Buss D. M., Barnes M. (2015). Preferences in human mate selection. Journal of Personality & Social Psychology, 50, 559–570. [Google Scholar]
- Buss D. M., Schmitt D. P. (1993). Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review, 100, 204–232. [DOI] [PubMed] [Google Scholar]
- Cárdenas R. A., Harris L. J. (2007). Do women’s preferences for symmetry change across the menstrual cycle? Evolution & Human Behavior, 28, 96–105. [Google Scholar]
- Chang Y., Xu J., Shi N., Zhang B., Zhao L. (2010). Dysfunction of processing task-irrelevant emotional faces in major depressive disorder patients revealed by expression-related visual MMN. Neuroscience Letters, 472, 33–37. [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Thomas A., Smith S. E., Aguirre G. K. (2009). The neural response to facial attractiveness. Neuropsychology, 23, 135–143. [DOI] [PubMed] [Google Scholar]
- Choi D., Egashira Y., Takakura J., Motoi M., Nishimura T., Watanuki S. (2015). Gender difference in N170 elicited under oddball task. Journal of Physiological Anthropology, 34, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J., Heatherton T. F., Whalen P. J., Kelley W. M. (2008). Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience, 20, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobey K. D., Little A. C., Roberts S. C. (2015). Hormonal effects on women’s facial masculinity preferences: The influence of pregnancy, post-partum, and hormonal contraceptive use. Biological Psychology, 104, 35–40. [DOI] [PubMed] [Google Scholar]
- Compton R. J. (2003). The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behavioral & Cognitive Neuroscience Reviews, 2, 115–129. [DOI] [PubMed] [Google Scholar]
- Conroybeam D., Buss D. M., Pham M. N., Shackelford T. K. (2015). How sexually dimorphic are human mate preferences? Personality & Social Psychology Bulletin, 41, 1082–1093. [DOI] [PubMed] [Google Scholar]
- Czigler I., Balázs L., Pató L. G. (2004). Visual change detection: Event-related potentials are dependent on stimulus location in humans. Neuroscience Letters, 364, 149–153. [DOI] [PubMed] [Google Scholar]
- Duncan L. A., Park J. H., Faulkner J., Schaller M., Neuberg S. L., Kenrick D. T. (2007). Adaptive allocation of attention: Effects of sex and sociosexuality on visual attention to attractive opposite-sex faces. Evolution & Human Behavior Official Journal of the Human Behavior & Evolution Society, 28, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante K. M., Griskevicius V., Simpson J. A., Cantú S. M., Li N. P. (2012). Ovulation leads women to perceive sexy cads as good dads. Journal of Personality & Social Psychology, 103, 292–305. [DOI] [PubMed] [Google Scholar]
- Duuren M. V., Kendell-Scott L., Stark N. (2003). Early aesthetic choices: Infant preferences for attractive premature infant faces. International Journal of Behavioral Development, 27, 212–219. [Google Scholar]
- Fink B., Penton-Voak I. (2002). Evolutionary psychology of facial attractiveness. Current Directions in Psychological Science, 11, 154–158. [Google Scholar]
- Gábor S., Motohiro K., István C. (2011). Visual mismatch negativity reveals automatic detection of sequential regularity violation. Frontiers in Human Neuroscience, 5, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski P. D., Schlegel K., Stoerig P. (2009). Effects of human race and face inversion on the N170. Journal of Psychophysiology, 22, 157–165. [Google Scholar]
- Gallup G. G., Jr, Frederick D. A. (2010). The science of sex appeal: An evolutionary perspective. Review of General Psychology, 14, 240–250. [Google Scholar]
- Gangestad S. W., Thornhill R. (1998). Menstrual cycle variation in women’s preferences for the scent of symmetrical men. Proceedings Biological Sciences, 265, 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S. W., Thornhill R., Garver-Apgar C. E. (2005). Adaptations to Ovulation. Hoboken, NJ: John Wiley. [Google Scholar]
- Gangestad S. W., Thornhill R., Garver-Apgar C. E. (2010). Men’s facial masculinity predicts changes in their female partners’ sexual interests across the ovulatory cycle, whereas men’s intelligence does not. Evolution and Human Behavior, 31, 412–424. [Google Scholar]
- Garverapgar C. E., Gangestad S. W., Simpson J. A. (2007). Women’s perceptions of men’s sexual coerciveness change across the menstrual cycle. Acta Psychologica Sinica, 39, 536–540. [Google Scholar]
- Gildersleeve K., Haselton M. G., Fales M. R. (2014). Meta-analyses and p-curves support robust cycle shifts in women’s mate preferences: Reply to Wood and Carden (2014) and Harris, Pashler, and Mickes (2014). Psychological Bulletin, 140, 1272–1280. [DOI] [PubMed] [Google Scholar]
- Gonzales J. E., Ferrer E. (2015). Efficacy of methods for ovulation estimation and their effect on the statistical detection of ovulation-linked behavioral fluctuations. Behavior Research Methods, 48, 1–20. [DOI] [PubMed] [Google Scholar]
- Gueguen N. (2009). The receptivity of women to courtship solicitation across the menstrual cycle: A field experiment. Biological Psychology, 80, 321–324. [DOI] [PubMed] [Google Scholar]
- Hahn A. C., Perrett D. I. (2014). Neural and behavioral responses to attractiveness in adult and infant faces. Neuroscience & Biobehavioral Reviews, 46, 591–603. [DOI] [PubMed] [Google Scholar]
- Hahn A. C., Symons L. A., Kredel T., Hanson K., Hodgson L., Schiavone L., Jantzen K. J. (2016). Early and late event-related potentials are modulated by infant and adult faces of high and low attractiveness. Social Neuroscience, 11, 207–220. [DOI] [PubMed] [Google Scholar]
- Harris C. R. (2011). Menstrual cycle and facial preferences reconsidered. Sex Roles, 64, 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. R. (2013). Shifts in masculinity preferences across the menstrual cycle: Still not there. Sex Roles, 69, 507–515. [Google Scholar]
- Henderson J. J. A., Anglin J. M. (2003). Facial attractiveness predicts longevity. Evolution and human behavior, 24, 351–356. [Google Scholar]
- Hooff J. C., Crawford H., Vugt M. (2011). The wandering mind of men: ERP evidence for gender differences in attention bias towards attractive opposite sex faces. Social Cognitive & Affective Neuroscience, 6, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoss R. A., Ramsey J. L., Griffin A. M., Langlois J. H. (2005). The role of facial attractiveness and facial masculinity/femininity in sex classification of faces. Perception, 34, 1459–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G., Fox C. J., Waite C. T., Aharon I., Barton J. J. (2008). The contribution of the fusiform gyrus and superior temporal sulcus in processing facial attractiveness: Neuropsychological and neuroimaging evidence. Neuroscience, 155, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A. (2007). Sex, beauty and the orbitofrontal cortex. International Journal of Psychophysiology, 63, 181–185. [DOI] [PubMed] [Google Scholar]
- Japee S., Crocker L., Carver F., Pessoa L., Ungerleider L. G. (2009). Individual differences in valence modulation of face-selective m170 response. Emotion, 9, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston V. S., Hagel R., Franklin M., Fink B., Grammer K. (2001). Male facial attractiveness: Evidence for hormone-mediated adaptive design. Evolution & Human Behavior, 22, 251–267. [Google Scholar]
- Jones B. C., Little A. C., Boothroyd L., Debruine L. M., Feinberg D. R., Smith M. J.…Perrett D. I. (2005). Commitment to relationships and preferences for femininity and apparent health in faces are strongest on days of the menstrual cycle when progesterone level is high. Hormones & Behavior, 48, 283–290. [DOI] [PubMed] [Google Scholar]
- Jones E. (2018). The early development of female sexuality. In Grigg R. (Ed), Female Sexuality (pp. 133–145). London, UK: Routledge. [Google Scholar]
- Jones B. C., Vukovic J., Little A. C., Roberts S. C., Debruine L. M. (2011). Circum-menopausal changes in women’s preferences for sexually dimorphic shape cues in peer-aged faces. Biological Psychology, 87, 453–455. [DOI] [PubMed] [Google Scholar]
- Jung K., Ruthruff E., Tybur J. M., Gaspelin N., Miller G. (2012). Perception of facial attractiveness requires some attentional resources: Implications for the “automaticity” of psychological adaptations. Evolution & Human Behavior, 33, 241–250. [Google Scholar]
- Kampe K. K., Frith C. D., Dolan R. J., Frith U. (2001). Reward value of attractiveness and gaze. Nature, 413, 589. [DOI] [PubMed] [Google Scholar]
- Kimura M., Katayama J., Ohira H., Schröger E. (2009). Visual mismatch negativity: New evidence from the equiprobable paradigm. Psychophysiology, 46, 402–409. [DOI] [PubMed] [Google Scholar]
- Kimura M., Schröger E., Czigler I. (2011). Visual mismatch negativity and its importance in visual cognitive sciences. Neuroreport, 22, 669–673. [DOI] [PubMed] [Google Scholar]
- Kokko H., Brooks R., Jennions M. D., Morley J. (2003). The evolution of mate choice and mating biases. Proceedings of the Royal Society of London B: Biological Sciences, 270, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kościński K. (2008). Facial attractiveness: Variation, adaptiveness and consequences of facial preferences. Anthropological Review, 71, 77–105. [Google Scholar]
- Kranz F., Ishai A. (2006). Face perception is modulated by sexual preference. Current Biology Cb, 16, 63–68. [DOI] [PubMed] [Google Scholar]
- Langlois J. H., Kalakanis L., Rubenstein A. J., Larson A., Hallam M., Smoot M. (2000). Maxims or myths of beauty? A meta-analytic and theoretical review. Psychological Bulletin, 126, 390–423. [DOI] [PubMed] [Google Scholar]
- Li N. P., Bailey J. M., Kenrick D. T., Linsenmeier J. A. (2002). The necessities and luxuries of mate preferences: Testing the tradeoffs. Journal of Personality and Social Psychology, 82(6), 947–955. [PubMed] [Google Scholar]
- Li N. P., Kenrick D. T. (2006). Sex similarities and differences in preferences for short-term mates: What, whether, and why. Journal of Personality and Social Psychology, 90, 468–489. [DOI] [PubMed] [Google Scholar]
- Li N. P., Yong J. C., Tov W., Sng O., Fletcher G. J., Valentine K. A.…Balliet D. (2013). Mate preferences do predict attraction and choices in the early stages of mate selection. Journal of Personality and Social Psychology, 105, 757–776. [DOI] [PubMed] [Google Scholar]
- Little A. C., Debruine L. M., Jones B. C. (2011). Exposure to visual cues of pathogen contagion changes preferences for masculinity and symmetry in opposite-sex faces. Proceedings Biological Sciences, 278, 2032–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little A. C., Jones B. C., Burriss R. P. (2007). Preferences for masculinity in male bodies change across the menstrual cycle. Hormones and Behavior, 51, 633–639. [DOI] [PubMed] [Google Scholar]
- Little A. C., Jones B. C., Burt D. M., Perrett D. I. (2007). Preferences for symmetry in faces change across the menstrual cycle. Biological Psychology, 76, 209–216. [DOI] [PubMed] [Google Scholar]
- Little A. C., Jones B. C., Debruine L. M. (2008). Preferences for variation in masculinity in real male faces change across the menstrual cycle: Women prefer more masculine faces when they are more fertile. Personality & Individual Differences, 45, 478–482. [Google Scholar]
- Little A. C., Jones B. C., Debruine L. M. (2011). Facial attractiveness: Evolutionary based research. Philosophical Transactions of the Royal Society of London, 366, 1638–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little A. C., Jones B. C., Pentonvoak I. S., Burt D. M., Perrett D. I. (2002). Partnership status and the temporal context of relationships influence human female preferences for sexual dimorphism in male face shape. Proceedings Biological Sciences, 269, 1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Wang J., Wang L., Wang J., Qin J. (2014). Neural responses to cartoon facial attractiveness: An event-related potential study. 科学通报(英文版) [Neuroscience Bulletin], 30, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S. J. (2014). An introduction to the event-related potential technique. Cambridge, MA: MIT Press. [Google Scholar]
- Malo A. F., Roldan E. R. S., Garde J. J., Soler A. J., Vicente J., Gortazar C., Gomendio M. (2009). What does testosterone do for red deer males? Proceedings of the Royal Society of London B: Biological Sciences, 276, 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner J. K., Gailliot M. T., Dewall C. N. (2007). Adaptive attentional attunement: Evidence for mating-related perceptual bias. Evolution & Human Behavior, 28, 28–36. [Google Scholar]
- Marzi T., Viggiano M. P. (2010). When memory meets beauty: Insights from event-related potentials. Biological Psychology, 84, 192–205. [DOI] [PubMed] [Google Scholar]
- Mcdonald M. M., Asher B. D., Kerr N. L., Navarrete C. D. (2011). Fertility and intergroup bias in racial and minimal-group contexts: Evidence for shared architecture. Psychological Science, 22, 860–865. [DOI] [PubMed] [Google Scholar]
- Miner E. J., Shackelford T. K. (2010). Mate attraction, retention and expulsion. Psicothema, 22, 9–14. [PubMed] [Google Scholar]
- Montoya R. M., Horton R. S., Kirchner J. (2008). Is actual similarity necessary for attraction? A meta-analysis of actual and perceived similarity. Journal of Social & Personal Relationships, 25, 889–922. [Google Scholar]
- Muñoz-Reyes J. A., Pita M., Arjona M., Sanchez-Pages S., Turiegano E. (2014). Who is the fairest of them all? The independent effect of attractive features and self-perceived attractiveness on cooperation among women. Evolution and Human Behavior, 35, 118–125. [Google Scholar]
- Navarrete C. D., Fessler D. M., Fleischman D. S., Geyer J. (2009). Race bias tracks conception risk across the menstrual cycle. Psychological Science, 20, 661–665. [DOI] [PubMed] [Google Scholar]
- O’Doherty J., Winston J., Critchley H., Perrett D., Burt D. M., Dolan R. J. (2003). Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia, 41, 147–155. [DOI] [PubMed] [Google Scholar]
- Oda R., Okuda A., Takeda M., Hiraishi K. (2014). Provision or good genes? Menstrual cycle shifts in women’s preferences for short-term and long-term mates’ altruistic behavior. Evolutionary Psychology: An International Journal of Evolutionary Approaches to Psychology & Behavior, 12, 888–900. [PubMed] [Google Scholar]
- Oinonen K. A., Mazmanian D. (2007). Facial symmetry detection ability changes across the menstrual cycle. Biological Psychology, 75, 136–145. [DOI] [PubMed] [Google Scholar]
- Palermo R., Rhodes G. (2007). Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia, 45, 75–92. [DOI] [PubMed] [Google Scholar]
- Perrett D. (2012). In your face: The new science of human attraction. New York, NY: Palgrave Macmillan. [Google Scholar]
- Pawlowski B., Jasienska G. (2005). Women’s preferences for sexual dimorphism in height depend on menstrual cycle phase and expected duration of relationship. Biological Psychology, 70, 38–43. [DOI] [PubMed] [Google Scholar]
- Pazo-Alvarez P., Amenedo E., Lorenzo-López L., Cadaveira F. (2004). Effects of stimulus location on automatic detection of changes in motion direction in the human brain. Neuroscience Letters, 371, 111–116. [DOI] [PubMed] [Google Scholar]
- Penton-Voak I. S., Jacobson A., Trivers R. (2004). Populational differences in attractiveness judgments of male and female faces: Comparing British and Jamaican samples. Evolution & Human Behavior, 25, 355–370. [Google Scholar]
- Penton-Voak I. S., Little A. C., Jones B. C., Burt D. M., Tiddeman B. P., Perrett D. I. (2003). Female condition influences preferences for sexual dimorphism in faces of male humans (Homo sapiens). Journal of Comparative Psychology, 117, 264–271. [DOI] [PubMed] [Google Scholar]
- Penton-Voak I. S., Perrett D. I. (2000). Female preference for male faces changes cyclically: Further evidence. Evolution & Human Behavior, 21, 39–48. [Google Scholar]
- Penton-Voak I. S., Perrett D. I., Castles D. L., Kobayashi T., Burt D. M., Murray L. K., Minamisawa R. (1999). Menstrual cycle alters face preference. Nature, 399, 741–742. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D. A., Lehmann D., Hendrick A. M., Regard M., Pascual-Marqui R. D., Davidson R. J. (2002). Affective judgments of faces modulate early activity (∼160 ms) within the fusiform gyri. Neuroimage, 16, 663–677. [DOI] [PubMed] [Google Scholar]
- Preston B. T., Stevenson I. R., Pemberton J. M., Coltman D. W., Wilson K. (2003). Overt and covert competition in a promiscuous mammal: The importance of weaponry and testes size to male reproductive success. Proceedings of the Royal Society of London B: Biological Sciences, 270, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Yang X., Qiao Z., Wang L., Ning N., Shi J.…Yang Y. (2011). Impairment in processing visual information at the pre-attentive stage in patients with a major depressive disorder: A visual mismatch negativity study. Neuroscience Letters, 491, 53–57. [DOI] [PubMed] [Google Scholar]
- Rhodes G. (2006). The evolutionary psychology of facial beauty. Annual Review of Psychology, 57, 199–226. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Lee K., Palermo R., Weiss M., Yoshikawa S., Clissa P.…Jeffery L. (2005). Attractiveness of own-race, other-race, and mixed-race faces. Perception, 34, 319–340. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Morley G., Simmons L. W. (2013). Women can judge sexual unfaithfulness from unfamiliar men’s faces. Biology letters, 9, 20120908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G., Yoshikawa S., Clark A., Lee K., Mckay R., Akamatsu S. (2001). Attractiveness of facial averageness and symmetry in non-Western cultures: In search of biologically based standards of beauty. Perception, 30, 611–625. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Zebrowitz L. A. (2002). Facial attractiveness: Evolutionary, cognitive, and social perspectives. Physical Review B Condensed Matter, 39, 459–466. [Google Scholar]
- Roberts S. C., Little A. C. (2008). Good genes, complementary genes and human mate preferences. Genetica, 132, 309–321. [DOI] [PubMed] [Google Scholar]
- Röder S., Brewer G., Fink B. (2009). Menstrual cycle shifts in women’s self-perception and motivation: A daily report method. Personality & Individual Differences, 47, 616–619. [Google Scholar]
- Schacht A., Werheid K., Sommer W. (2008). The appraisal of facial beauty is rapid but not mandatory. Cognitive Affective & Behavioral Neuroscience, 8, 132–142. [DOI] [PubMed] [Google Scholar]
- Semlitsch H. V., Anderer P., Schuster P., Presslich O. (1986). A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology, 23, 695–703. [DOI] [PubMed] [Google Scholar]
- Senior C. (2003). Beauty in the brain of the beholder. Neuron, 38, 525–528. [DOI] [PubMed] [Google Scholar]
- Slater A., Quinn P. C., Hayes R., Brown E. (2000). The role of facial orientation in newborn infants’ preference for attractive faces. Developmental Science, 3, 181–185. [Google Scholar]
- Smith F. G., Debruine L. M., Jones B. C., Krupp D. B., Welling L. L. M., Conway C. A. (2009). Attractiveness qualifies the effect of observation on trusting behavior in an economic game. Evolution and Human Behavior, 30, 393–397. [Google Scholar]
- Stefanics G., Csukly G., Komlósi S., Czobor P., Czigler I. (2012). Processing of unattended facial emotions: a visual mismatch negativity study. Neuroimage, 59, 3042–3049. [DOI] [PubMed] [Google Scholar]
- Stefanics G., Kimura M., Czigler I. (2011). Visual mismatch negativity reveals automatic detection of sequential regularity violation. Frontiers in Human Neuroscience, 5, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanics G., Kremláček J., Czigler I. (2014). Visual mismatch negativity: A predictive coding view. Frontiers in Human Neuroscience, 8, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susac A., Ilmoniemi R. J., Pihko E., Ranken D., Supek S. (2010). Early cortical responses are sensitive to changes in face stimuli. Brain research, 1346, 155–164. [DOI] [PubMed] [Google Scholar]
- Susac A., Ilmoniemi R. J., Pihko E., Supek S. (2004). Neurodynamic studies on emotional and inverted faces in an oddball paradigm. Brain Topography, 16, 265–268. [DOI] [PubMed] [Google Scholar]
- Thornhill R., Gangestad S. W., Moller A. P. (1999). The biological significance of fluctuating asymmetry and sexual selection: A reply to palmer. American Naturalist, 154, 234–241. [DOI] [PubMed] [Google Scholar]
- Thornhill R., Palmer C. T. (2000). A natural history of rape: Biological bases of sexual coercion. Biology & Philosophy, 17, 445–456. [Google Scholar]
- Tsukiura T., Cabeza R. (2011). Shared brain activity for aesthetic and moral judgments: Implications for the Beauty-is-Good stereotype. Social Cognitive & Affective Neuroscience, 6, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlamings P. H., Goffaux V., Kemner C. (2009). Is the early modulation of brain activity by fearful facial expressions primarily mediated by coarse low spatial frequency information? Journal of vision, 9, 12–12. [DOI] [PubMed] [Google Scholar]
- Wang H., Hahn A. C., Fisher C. I., Debruine L. M., Jones B. C. (2014). Women’s hormone levels modulate the motivational salience of facial attractiveness and sexual dimorphism. Psychoneuroendocrinology, 50, 246–251. [DOI] [PubMed] [Google Scholar]
- Wei J. H., Chan T. C., Luo Y. J. (2002). A modified oddball paradigm “cross-modal delayed response” and the research on mismatch negativity. Brain Research Bulletin, 57, 221–230. [DOI] [PubMed] [Google Scholar]
- Welling L. L., Jones B. C., DeBruine L. M., Conway C. A., Smith M. L., Little A. C., Al-Dujaili E. A. (2007). Raised salivary testosterone in women is associated with increased attraction to masculine faces. Hormones and Behavior, 52, 156–161. [DOI] [PubMed] [Google Scholar]
- Werheid K., Schacht A., Sommer W. (2007). Facial attractiveness modulates early and late event-related brain potentials. Biological Psychology, 76, 100–108. [DOI] [PubMed] [Google Scholar]
- Wilson M., Daly M. (2004). Do pretty women inspire men to discount the future? Proceedings of Biological Science, 271, S177–S179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J. S., O’Doherty J., Kilner J. M., Perrett D. I., Dolan R. J. (2007). Brain systems for assessing facial attractiveness. Neuropsychologia, 45, 195–206. [DOI] [PubMed] [Google Scholar]
- Wood W., Carden L. (2014). Elusiveness of menstrual cycle effects on mate preferences: Comment on Gildersleeve, Haselton, and Fales (2014). Psychological Bulletin, 140, 1265–1271. [DOI] [PubMed] [Google Scholar]
- Wronka E., Walentowska W. (2011). Attention modulates emotional expression processing. Psychophysiology, 48, 1047–1056. [DOI] [PubMed] [Google Scholar]
- Yan Z., Wei B., Zhao P., Zheng M., Zhang L. (2016). Gender differences in memory processing of female facial attractiveness: Evidence from event-related potentials. Neurocase, 22, 317–323. [DOI] [PubMed] [Google Scholar]
- Young J. A., Critelli J. W., Keith K. W. (2005). Male age preferences for short-term and long-term mating. Sexualities Evolution & Gender, 7, 83–93. [Google Scholar]
- Zhang Z., Deng Z. (2012). Gender, facial attractiveness, and early and late event-related potential components. Journal of Integrative Neuroscience, 11, 477–487. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kong F., Chen H., Jackson T., Han L., Meng J.…Najam A. (2011). Identifying cognitive preferences for attractive female faces: An event-related potential experiment using a study-test paradigm. Journal of Neuroscience Research, 89, 1887–1893. [DOI] [PubMed] [Google Scholar]
- Zhao L., Li J. (2006). Visual mismatch negativity elicited by facial expressions under non-attentional condition. Neuroscience letters, 410, 126–131. [DOI] [PubMed] [Google Scholar]
- Zietsch B. P., Lee A. J., Sherlock J. M., Jern P. (2015). Variation in women’s preferences regarding male facial masculinity is better explained by genetic differences than by previously identified context-dependent effects. Psychological Science, 26, 1440–1448. [DOI] [PubMed] [Google Scholar]