FIGURE 6.
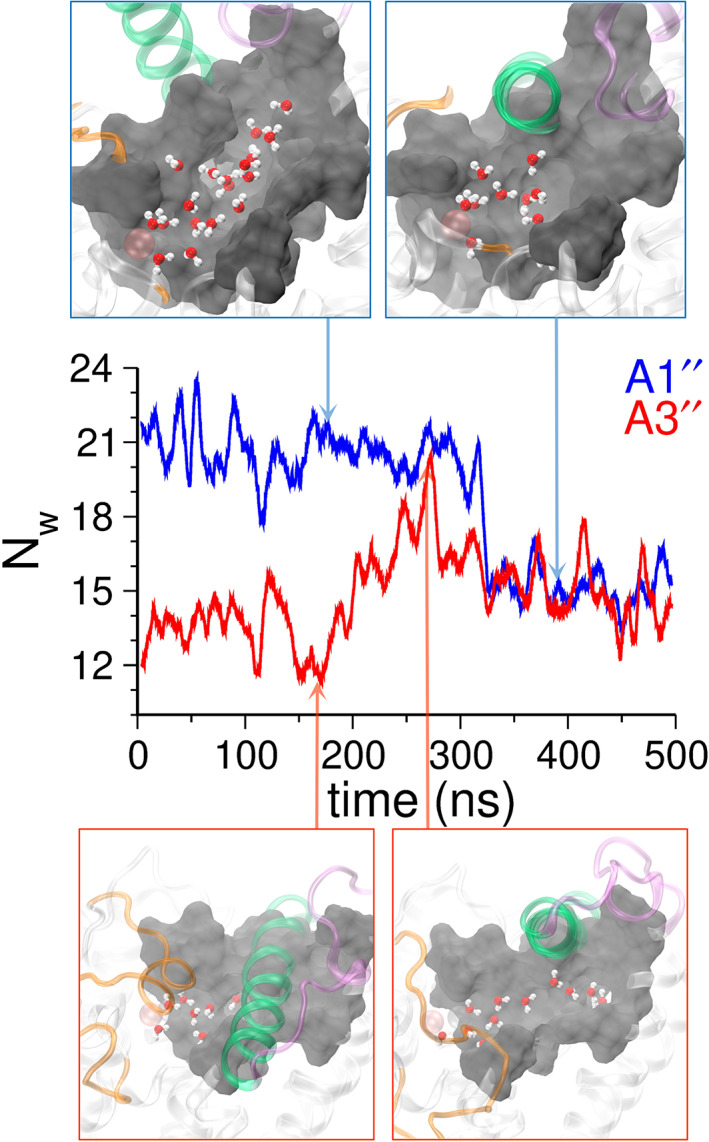
The number of water molecules (N w) in the catalytic site of PDE5. N w was calculated based on the number of water molecules within 8 Å of the Asp764:O or Leu765:C γ or ILE768:C γ atoms of the residues in the PDE5 catalytic site. Shown are the traces of N w for the A1″ (blue) and A3″ (red) simulations. In A1″ simulation, the α14 helix makes a transition from an outward (N w = ~20) to an inward state (N w = ~14). The representative structures highlighting the α14 helix (green) in the outward state and the inward state are shown in blue boxes. In the A3″ simulation, the inward state of the α14 helix has inaccessible catalytic site (N w = ~12). However, due to the complete unfolding of the H‐loop (yellow) and the outward motion of the α14 helix, the catalytic site of PDE5 opens and N w increases (~20). In the catalytic site of PDE5, the H‐loop (yellow), the α14 helix (green), and the M‐loop (purple) are shown in cartoon representations, and the water molecules are shown in CPK representations (oxygen atoms in red and hydrogen atoms in white). The zinc (gray) and magnesium (pink) ions are shown as spheres. The N w traces of all apo and evodiamine‐bound simulations are shown in Figure S17.
