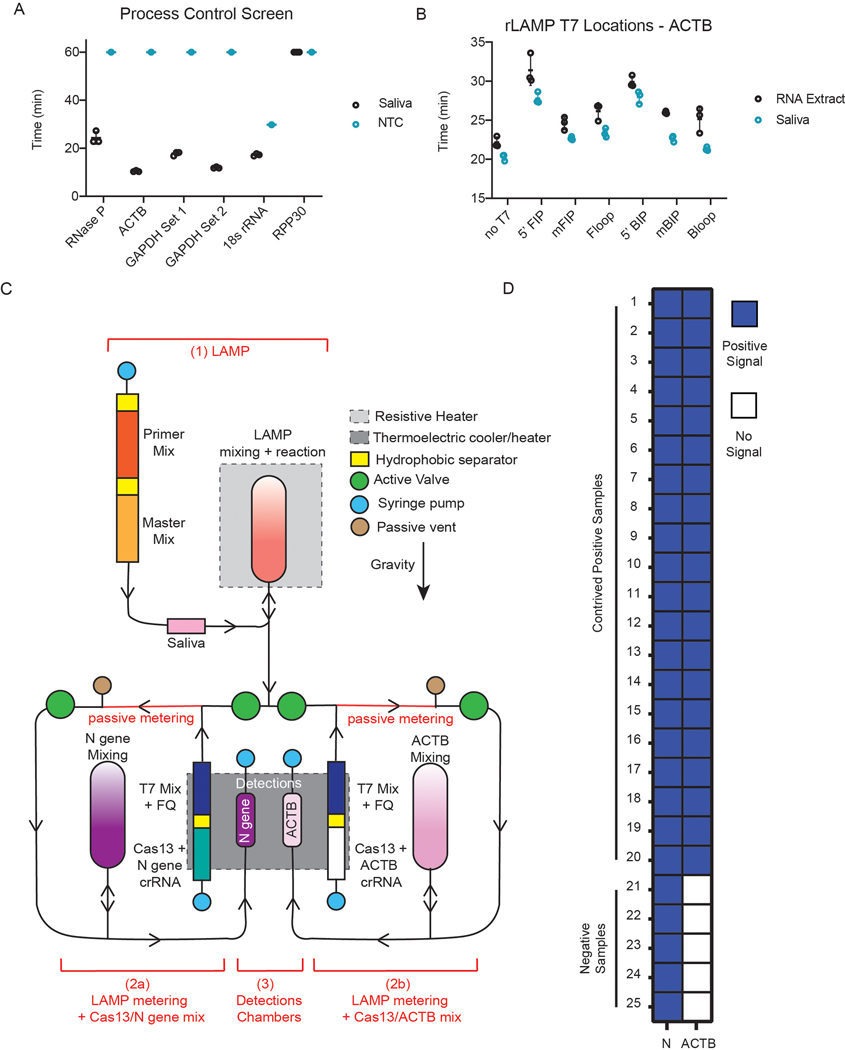
Extended Data Fig. 2 |. Validation of the microfluidic system.
(a) Screen of process control primer sets on commercial saliva samples. (b) Screen of T7 promoter locations on ACTB primer set on RNA extract and commercial saliva samples. (c) Schematic of cartridge design. The reaction can be separated in three steps: 1. amplification reaction; 2. post-amplification metering + Cas13 mix; 3. Cas13 reaction in detection chambers. Reagents stored on the cartridge are separated via a proprietary hydrophobic solution to avoid premature initiation of the reactions. After the LAMP reaction, the sample is split into two reactions: The left part of the cartridge will expose the sample to N gene crRNA (step 2a) while the right side of the cartridge will act as internal control with only ACTB crRNA (step 2b). (d) Results of on-board DISCoVER on 25 individual saliva samples (20 samples with SARS-CoV-2 genomic RNA at 500 cp/μL, 5 expected negative samples). A 2-fold fluorescence increase over blank cartridges at 10 minutes was the experimentally determined criteria for positive results. Samples with value below this threshold at 10 minutes were declared negative.