Abstract
Introduction
Parathyroid hormone-lowering responses after administration of three different therapies capable of raising serum total 25-hydroxyvitamin D (25OHD) were evaluated in patients with secondary hyperparathyroidism (SHPT), vitamin D insufficiency (VDI), and stage 3 or 4 chronic kidney disease (CKD).
Methods
Sixty-nine adult subjects with intact parathyroid hormone (iPTH) ≥85 and <500 pg/mL and VDI (25OHD <30 ng/mL) were randomized after ≥4-week washout to 2 months of open-label treatment with: (1) extended-release calcifediol (ERC) 60 μg/day; (2) immediate-release calcifediol (IRC) 266 μg/month; (3) high-dose cholecalciferol (HDC) 300,000 IU/month; or (4) paricalcitol plus low-dose cholecalciferol (PLDC) 1 or 2 μg and 800 IU/day, used as reference hormone replacement therapy. Serum 25OHD, calcium (Ca), phosphorus (P), plasma iPTH, and adverse events were monitored weekly. No clinically significant differences were observed at baseline between treatment groups.
Results
Sixty-two subjects completed the study per protocol (PP; 14–17 per group). Mean (SD) 25OHD and iPTH at baseline were 20.6 (6.6) ng/mL and 144.8 (90) pg/mL, respectively. Mean 25OHD increased at end of treatment (EOT) to 82.9 (17.0) ng/mL with ERC (p < 0.001) and 30.8 (11.6) ng/mL with HDC (p < 0.05), but remained unchanged with IRC and PLDC. EOT 25OHD levels reached ≥30 ng/mL in all subjects treated with ERC and in 44% with HDC. All subjects attained EOT 25OHD levels ≥50 ng/mL with ERC versus none with other therapies. iPTH response rates at EOT (≥10, 20 or 30% below baseline) were similar for ERC and PLDC; rates for IRC and HDC were much lower. No significant changes from baseline were observed in ionized or corrected total Ca or P in any group. One episode of hypercalcemia (>10.3 mg/dL) occurred with PLDC. Hyperphosphatemia (>5.5 mg/dL) occurred once with ERC, eight times with HDC, 3 times with IRC, and twice with PLDC.
Conclusion
ERC was highly effective in both raising serum 25OHD and decreasing iPTH in patients with SHPT, VDI, and stage 3 or 4 CKD. iPTH-lowering response rates with ERC were similar to daily PLDC, the reference therapy; rates with IRC or HDC were significantly lower. ERC is an attractive alternative to vitamin D hormone therapy in CKD patients.
Keywords: Vitamin D, Cholecalciferol, Calcifediol, Secondary hyperparathyroidism, Chronic kidney disease, Extended-release calcifediol
Introduction
Cholecalciferol or ergocalciferol supplementation is recommended by both the Kidney Disease Outcomes Quality Initiative (K/DOQI) and Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines [1–3] to address secondary hyperparathyroidism (SHPT) arising from vitamin D insufficiency (VDI) in patients with chronic kidney disease (CKD). Supplementation is widely used despite a lack of expert consensus regarding its effectiveness for lowering parathyroid hormone (PTH) levels, the appropriate target for serum total 25-hydroxyvitamin D (25OHD), and the optimal dosing regimen [3–6]. In the USA and certain European countries, extended-release calcifediol (ERC) is approved to treat SHPT in adults with VDI and stage 3–4 CKD at a dose of 30 μg per day escalating, as needed, to 60 μg per day. Immediate-release calcifediol (IRC) is approved in some European countries to treat VDI associated with renal osteodystrophy at a dose of 266 μg per month and to treat rickets, osteomalacia, and hypocalcemia with lower and different dosing regimens.
In CKD patients, vitamin D supplementation is often replaced or combined with oral calcitriol (or other 1α-hydroxylated vitamin D analog) therapy when PTH levels rise persistently above the normal range with advancing disease. The original KDIGO clinical practice guideline for the treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) recommended this approach in patients with stage 3 or 4 CKD [2], consistent with the widely held view that administration of 1α-hydroxylated drugs becomes necessary in later stages of CKD. The more recent KDIGO guideline update [3] reversed this recommendation, suggesting instead that hormone replacement should no longer be routinely used in nondialysis CKD due to increased risk of hypercalcemia. The updated KDIGO guideline also highlighted the unproven effectiveness of supplements for suppressing elevated PTH, leaving nephrologists without clear guidance on how best to treat SHPT in CKD patients. More recent evidence [6, 7] has confirmed the limited effectiveness of vitamin D supplements and supports the alternative use of ERC. The present randomized controlled trial (RCT) evaluated PTH-lowering responses to oral ERC, IRC, and high-dose cholecalciferol (HDC) in relation to oral paricalcitol plus low-dose cholecalciferol (PLDC), used as a reference vitamin D hormone therapy, in patients with stage 3 or 4 CKD and VDI.
Methods
Study Design
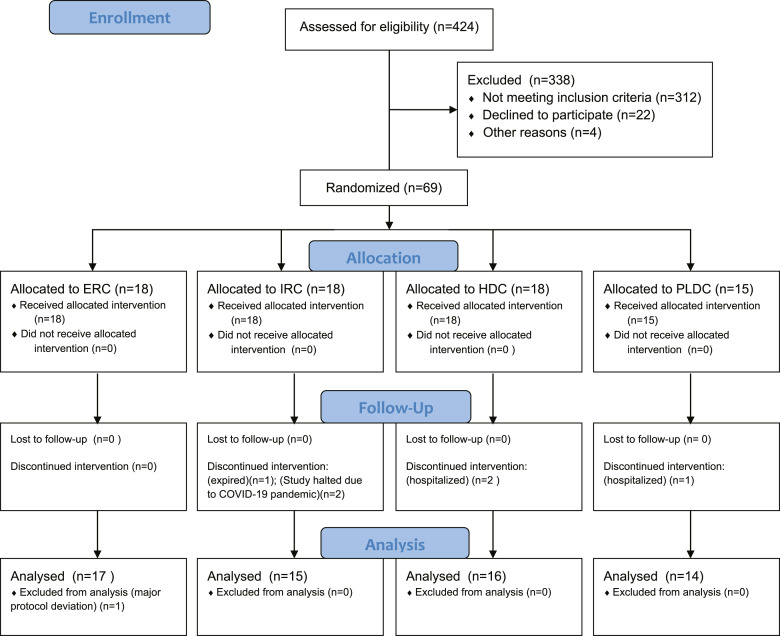
Four hundred and twenty-four adult patients were assessed for eligibility, and sixty-nine with SHPT, VDI, and stage 3 or 4 CKD (target of 80) were enrolled (Fig. 1) from 5 US sites and randomized 1:1:1:1, balanced for body weight, to receive 8 weeks of open-label treatment with ERC, IRC, HDC, or PLDC, which served as a reference therapy. The study began in August 2018 and terminated prematurely in April 2020 due to potential risks to subject health imposed by the intensifying coronavirus disease 2019 (COVID-19) pandemic. The primary pharmacodynamic (PD) endpoints of interest included absolute and relative changes in serum total 25OHD and plasma intact parathyroid hormone (iPTH). Secondary PD endpoints of interest included absolute and relative changes in serum total 1,25-dihydroxyvitamin D (1,25(OH)2D), paricalcitol, and 24,25-dihydroxyvitamin D3 (24,25(OH)2D3). Safety was assessed by changes in serum calcium (Ca), phosphorus (P), estimated glomerular filtration rate (eGFR) and by treatment-emergent adverse events (TEAEs).
Fig. 1.
Consort diagram.
Subjects were housed in a phase 1 unit for 14–26 h on day 1 (start of dosing) and again on day 29 (start of second month of dosing) to allow intensive blood sampling required for pharmacokinetic (PK) and PD analyses. Blood samples were collected as follows: in the phase 1 unit at −2, 0, 2, 4, 6, and 12 h; at 24 h (either in the phase 1 unit or during an outpatient visit); at 48 h; and, subsequently, at weekly intervals. Collected samples were analyzed for plasma iPTH and serum ionized calcium, total Ca, P, and the following vitamin D metabolites: total 25OHD, calcifediol (25OHD3), 24,25(OH)2D3, and total 1,25(OH)2D. Serum total Ca values were corrected for albumin below 4.0 g/dL. The Modification of Diet in Renal Disease equation [8] was used to calculate eGFR.
Subject Selection
Eligible subjects were ≥18 years of age and had CKD (not requiring regular dialysis) with an eGFR of ≥15 and <60 mL/min/1.73m2. Other inclusion criteria included serum total 25OHD <30 ng/mL (after discontinuation and a ≥4-week washout of nonstudy vitamin D therapy), plasma iPTH ≥85 and <500 pg/mL (if not receiving calcitriol or 1α-hydroxylated analog), serum total Ca <9.8 mg/dL, and serum P <5.5 mg/dL. Exclusion criteria included history of serum total Ca ≥9.8 mg/dL or serum P ≥5.5 mg/dL, need for phosphate binders to maintain serum P <5.5 mg/dL, use of calcimimetic therapy within 12 weeks of screening, prior parathyroidectomy, and prior/planned kidney transplant. Bone metabolism or bisphosphonate therapies must have been discontinued for at least 1 year prior to enrollment.
Drug Products and Dosing
Subjects were randomized to oral treatment with one of the three different therapies capable of raising serum total 25OHD: (1) ERC 60 μg/day; (2) IRC 266 μg/month; (3) HDC 300,000 IU/month; or (4) PLDC, a reference therapy consisting of 1 μg of paricalcitol (which is incapable of raising serum 25OHD) and 800 IU/day of cholecalciferol. ERC was provided as two extended-release capsules each containing 30 μg of calcifediol (Rayaldee®; OPKO Pharmaceuticals, Miami). IRC was provided as one immediate-release capsule containing 266 μg of calcifediol (Hidroferol®; FAES FARMA, Vizcaya). HDC was provided as six capsules each containing 50,000 IU cholecalciferol (InVita, Plettenberg). PLDC was provided as one or two capsules containing 1 μg of paricalcitol (AbbVie, Lake County or Zydus, Pennington) along with one capsule containing 800 IU of cholecalciferol (InVita). The strengths of all study drugs were verified analytically prior to use.
ERC and PLDC were administered in the phase 1 unit on days 1 and 29 after an overnight fast and before breakfast, and at home on all other study days (ERC at bedtime and PLDC in the morning). IRC and HDC were administered only in the phase 1 unit on days 1 and 29 after an overnight fast and before breakfast. Standardized meals were provided to all subjects while housed in the phase 1 unit. Paricalcitol dosing started at 1 μg/day and was increased to 2 μg/day on day 29 if plasma iPTH was not reduced by ≥30% and remained >70 pg/mL, serum total Ca was <9.8 mg/dL, and serum P was <5.5 mg/dL. Subjects in all groups reduced the assigned dose if they had confirmed plasma iPTH <30 pg/mL, serum total Ca >10.3 mg/dL, or serum P > 5.5 mg/dL. Subjects in all groups suspended dosing if plasma iPTH was confirmed <15 pg/mL or serum Ca >11.0 mg/dL. Dosing was resumed when plasma iPTH rose to ≥30 pg/mL and serum Ca decreased to <9.8 mg/dL.
Rationale for Selected Doses
It was admittedly difficult to choose dosing regimens that would satisfy all health care professionals given the current lack of consensus regarding either a target for serum total 25OHD in CKD patients or a dosing regimen by which vitamin D supplementation is best administered. ERC is approved in the USA and in numerous European countries for administration at 30 μg/day escalating, as needed, to 60 μg/day to achieve a targeted reduction in iPTH, but does not produce steady-state serum 25OHD levels at either dose until at least 3 months of treatment has been completed. A dose of 60 μg/day of ERC was selected to accelerate the timing under which serum total 25OHD targets of 30 and 50 ng/mL could be attained, as normal dose titration (which would have yielded similar 25OHD levels) required a 6-month treatment period. The 300,000 IU monthly dose of HDC was chosen because it is the highest dose of cholecalciferol routinely used and few nephrologists would recommend using a higher dose. The selected monthly dose of IRC (266 μg) is the recommended dose (per the Summary of Product Characteristics) for treating VDI or deficiency associated with renal osteodystrophy. IRC is not approved in the USA. PLDC was selected as the reference therapy because oral paricalcitol has been established as effective in reducing elevated plasma iPTH in stage 3 and 4 CKD and it was administered in accordance with the approved labeling. Administration of 800 IU/day of cholecalciferol has been deemed appropriate for treating VDI by the Institute of Medicine [9]. Paricalcitol was chosen instead of calcitriol because the 4-fold higher dosage (due to the established 4-fold lower binding affinity for the vitamin D receptor [10]) made analysis of postdose serum drug levels feasible.
Laboratory and Clinical Procedures
Plasma and serum samples were shipped on dry ice to the appropriate central lab for analysis. Plasma iPTH levels were determined at ARUP Laboratories (Salt Lake City) by electrochemiluminescence immunoassay (Roche Cobas). Serum total 25OHD was determined at ACL Laboratories (West Allis) by chemilluminescent immunoassay (ADVIA Centaur). Analyses of serum calcifediol, 24,25(OH)2D3, and paricalcitol were conducted at Syneos (Quebec City) using LC/MS/MS. Analysis of serum total 1,25(OH)2D was conducted at BioReference Laboratories (Elmwood Park).
Analysis of Data
Pretreatment “baseline” values were defined as the average of all postwashout (if required) measurements obtained prior to initiation of treatment on day 1. Values for the first efficacy assessment period (EAP1) were defined as the average of up to three measurements, one obtained on day 22 and two on day 29 (predose). Values for the second efficacy assessment period (EAP2) were defined as the average of up to two measurements, one obtained on each of days 50 and 57. Prespecified PK and PD endpoints (25OHD, iPTH, Ca, and P) were analyzed for the per-protocol (PP) population (n = 62, see below), which excluded 7 subjects who had a major protocol deviation or never reached EAP1 and/or EAP2 (see below). Statistical comparisons of changes from baseline to a specified time point were conducted using a t test. Other statistical comparisons between groups were conducted using ANOVA with Bonferroni’s correction or χ2 analysis, as appropriate. Safety endpoints were analyzed for all subjects who received at least one dose of study medication (n = 69).
Results
Subject Population
Sixty-two (90%) of the 69 enrolled subjects completed all 8 weeks of treatment and were included in the PP population and the analysis of data: 17 subjects received ERC treatment and 16, 15, and 14 subjects received HDC, IRC, and PLDC, respectively. Seven of the 69 subjects (10%) were excluded from analysis. One of these seven subjects was assigned to the ERC group and completed 8 weeks of treatment but was excluded due to a major protocol deviation arising from noncompliance with the prescribed medication. The other six subjects were excluded because they withdrew from the study prematurely and no data were obtained at either EAP1 or EAP2. The reasons why four of these six subjects withdrew are as follows: one subject in the PLDC group was hospitalized for intervertebral disc protrusion, lumbar spinal stenosis, and radiculopathy; two subjects in the HDC group were hospitalized for acalculous cholecystitis and hypertensive encephalopathy, respectively; one subject in the IRC group expired during the treatment period due to acute respiratory distress syndrome possibly related to COVID-19. After this death, the study was halted out of concern that further study visits might unnecessarily increase subjects' risk of exposure to SARS-CoV-2, causing two additional subjects assigned to IRC treatment to end participation prematurely.
Study Population
Baseline demographic data for each treatment group in the PP population are shown in Table 1. Forty-seven percent of subjects were female, 50% African-American or Black, 48% White, 2% Native American, and 6.5% Hispanic. Mean (SE) age at baseline was 67.2 (1.5) years (range 33–86), plasma iPTH was 144.8 (11.4) pg/mL, serum total 25OHD was 20.6 (0.8) ng/mL, serum total Ca was 9.1 (0.1) mg/dL, and serum P was 3.6 (0.1) mg/dL. Mean baseline body weight was 102.2 (3.1) kg, body mass index (BMI) was 35.1 kg/m2 (1.0), and eGFR was 31.9 (1.2) mL/min/1.73m2. No significant demographic or clinical differences were observed between treatment groups at baseline, except that the HDC group included fewer women (25%) than the ERC or PLDC groups, and had lower baseline serum Ca than the PLDC group (p < 0.05). The doses remained constant during the treatment period for ERC, IRC, and HDC, but the paricalcitol dose increased from 1 μg/day to 2 μg/day in 57% of subjects in the PLDC group.
Table 1.
Baseline demographics
| Treatment group | ERC | IRC | HDC | PLDC | Total |
|---|---|---|---|---|---|
| Number of subjects | 17 | 15 | 16 | 14 | 62 |
| Gender | |||||
| Female | 11* | 5 | 4 | 9* | 29 |
| Male | 6 | 10 | 12 | 5 | 33 |
| Race | |||||
| Black | 8 | 9 | 8 | 6 | 31 |
| White | 9 | 5 | 8 | 8 | 30 |
| Other | 0 | 1 | 0 | 0 | 1 |
| Weight, kga | 98.8 (7.3) | 102.7 (4.5) | 103.5 (6.2) | 104.1 (6.7) | 102.2 (3.1) |
| BMI, kg/m2 | 35.1 (2.0) | 34.4 (1.7) | 33.7 (1.7) | 37.7 (2.5) | 35.1 (1.0) |
| Age, years | 66.7 (2.6) | 71.4 (3.9) | 67.0 (2.4) | 63.4 (3.3) | 67.2 (1.5) |
| Serum Ca, mg/dL | 9.2 (0.1) | 9.2 (0.1) | 8.8 (0.2) | 9.2 (0.1)* | 9.1 (0.1) |
| Serum P, mg/dL | 3.7 (0.1) | 3.7 (0.1) | 3.6 (0.2) | 3.6 (0.1) | 3.6 (0.1) |
| Total 25D, ng/mL | 21.8 (1.6) | 22.7 (1.5) | 18.8 (1.9) | 18.7 (1.6) | 20.6 (0.8) |
| iPTH, pg/mL | 113.5 (8.5) | 137.9 (15.9) | 162.8 (24.6) | 169.7 (36.7) | 144.8 (11.4) |
| eGFR, mL/min/1.73 m2 | 33.6 (2.0) | 28.9 (2.0) | 29.6 (2.0) | 35.8 (3.2) | 31.9 (1.2) |
25D, 25-hydroxyvitamin D; BMI, body mass index; Ca, Calcium; eGFR, estimated glomerular filtration rate; iPTH, intact parathyroid hormone; P, phosphorus.
*Significantly different from HDC group, p < 0.05.
aBaseline data are mean (SE).
Serum Total 25OHD
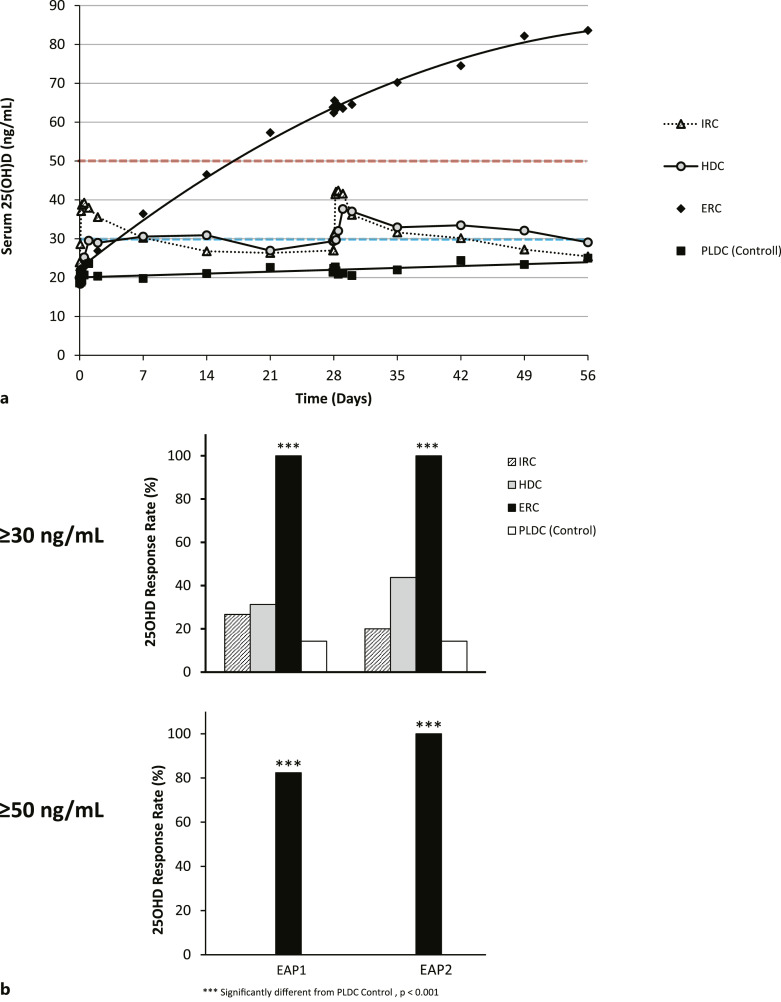
Mean serum total 25OHD increased gradually and continuously in the ERC group (Fig. 2a) surpassing 60 ng/mL by the end of the first month of treatment (EAP1) and 80 ng/mL by the end of the second month of treatment (EAP2). In the IRC group, mean 25OHD rose abruptly to the 37–43 ng/mL range and then declined to levels below 30 ng/mL about 2 weeks prior to the end of the dosing interval. In the HDC group, mean 25OHD levels rose to 30–40 ng/mL, with many subjects remaining well below this range, and then declined below 30 ng/mL before the dosing interval ended. Mean 25OHD levels with PLDC remained unchanged and well below 30 ng/mL.
Fig. 2.
a Time courses of changes in serum 25OHD levels. Mean serum total 25OHD levels (ng/mL) in the four treatment groups were plotted using available time point data, from the start to end of treatment. The targeted thresholds for serum 25OHD of 30 or 50 ng/mL are denoted by the horizontal dashed lines. b Serum 25OHD response rates. Two different thresholds were used to assess serum total 25OHD response rates: ≥30 ng/mL and ≥50 ng/mL. Response rates (percentage of subjects in each treatment group) were calculated using mean data obtained for each subject at EAP1 and at EAP2. Significant differences from the PLDC (reference) group are as indicated.
Using a threshold of 30 ng/mL, ERC treatment achieved a serum total 25OHD response rate of 100% in both EAP1 and EAP2, while IRC, HDC, and PLDC had corresponding response rates of less than 27%, 44%, and 15%, respectively (Fig. 2b). Using a target of 50 ng/mL, ERC achieved response rates above 80% in EAP1 and of 100% in EAP2, but no other therapy raised serum 25OHD to this same threshold (Fig. 2b) in any subject. Serum 25OHD levels achieved in EAP2 by subjects in all groups showed a significant inverse relationship with body weight and BMI.
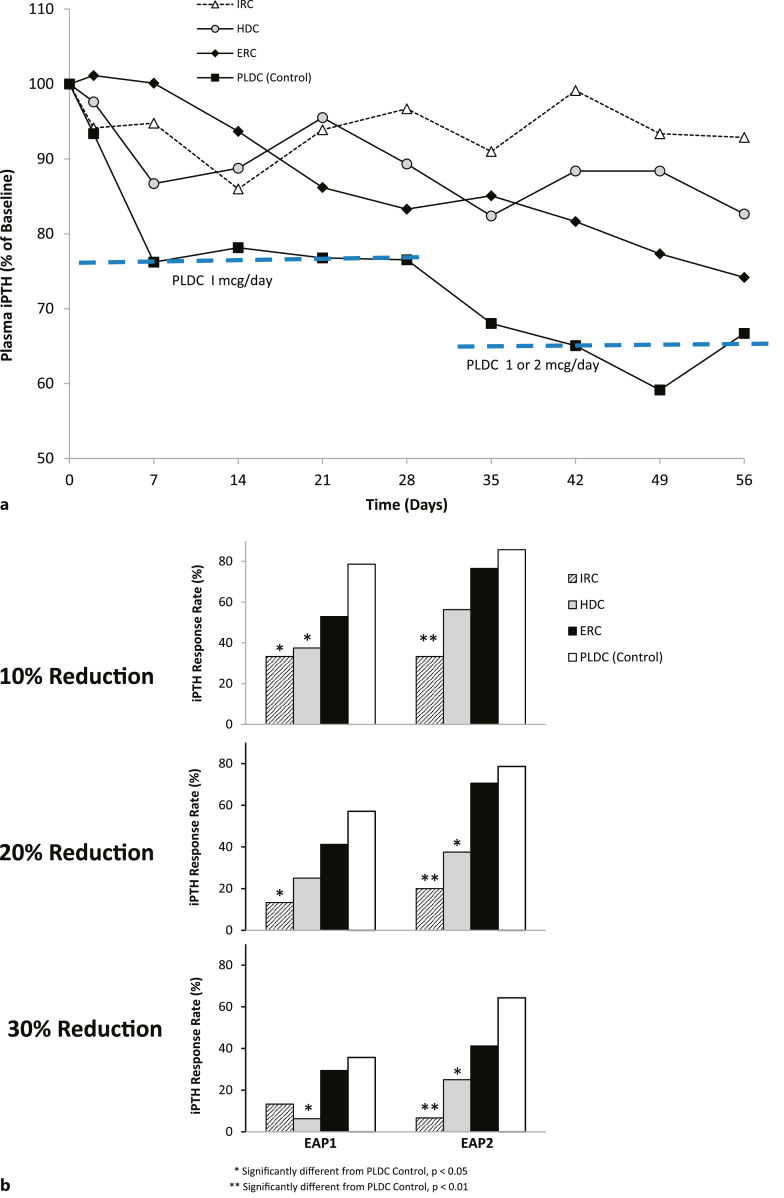
Plasma iPTH Levels
Reductions in iPTH, expressed as a percentage of baseline, are displayed over time for the four treatment groups in Figure 3a. ERC and PLDC treatment produced greater reductions in iPTH by EOT than either IRC or HDC treatment. Plasma iPTH-lowering responses observed in EAP1 and EAP2 are summarized for all four treatment groups in Figure 3b. Response rates for ERC in both EAP1 and EAP2 were directionally lower but did not differ significantly from those of PLDC irrespective of whether “response” was defined as a ≥10, 20, or 30% reduction from pretreatment baseline. Response rates with IRC and HDC in EAP2 were significantly lower (p < 0.05) than PLDC. Using the ≥10% threshold for defining “response,” ERC and PLDC had response rates in EAP2 of 76.5% and 85.7%, respectively, while IRC and HDC had rates of 33.1% and 56.3%, respectively. Using the ≥20% threshold, ERC and PLDC had response rates in EAP2 of 70.6% and 78.6%, respectively, compared to response rates of 20.0% and 37.5% for the IRC and HDC groups. Using the ≥30% threshold, ERC achieved a response rate in EAP2 of 41.2% compared with 64.3% for PLDC, 6.7% for IRC, and 25.0% for HDC.
Fig. 3.
a Time courses of changes in plasma iPTH levels. Mean serum total plasma iPTH levels (% of baseline) in the four treatment groups were plotted using available time point data, from the start to end of treatment. b Plasma iPTH response rates. Three different thresholds were used to assess iPTH-lowering response rates: ≥10%, ≥20%, and ≥30% reductions from baseline at EAP1 and at EAP2. Significant differences from the PLDC (reference) group are as indicated.
PK Data for Vitamin D Metabolites
Within the first 24 h after dosing on days 1 and 29, mean serum total 25OHD increased by small amounts with ERC and PLDC, and large amounts with IRC and HDC (Table 2). Mean serum 25OHD3 in all treatment groups showed essentially the same changes that were observed for serum 25OHD. Corresponding increases in serum total 1,25(OH)2D during these periods were small for ERC and large for IRC and HDC. Serum paricalcitol levels rose from undetectable levels to 19.9 pg/mL following dosing on day 1 and from 19.6 to 51.5 pg/mL (263%) after dosing on day 29, and these increases were presumably offset to some degree by compensatory decreases in serum 1,25(OH)2D. Sudden increases in serum 24,25(OH)2D3 of 17.8% and 20.5% were observed in the 24-h postdose periods on days 1 and 29 for IRC; corresponding increases with ERC, HDC, and PLDC were much lower (<6.8%). Gradual increases in serum 24,25(OH)2D3 were observed over time with ERC administration in fixed proportion to the elevations in serum 25OHD.
Table 2.
Single- and repeated-dose PK data for vitamin D metabolites
| Treatment | Day 1 dose | Day 29 dose | ||||
|---|---|---|---|---|---|---|
| Baseline | C max | % change | Baseline | C max | % change | |
| Changes in serum 25OHD, ng/mL | ||||||
| ERC | 22.7 | 24.1 | 6.3 | 63.1 | 65.5 | 3.8 |
| IRC | 23.3 | 39.3 | 68.8 | 27.0 | 42.4 | 57.1 |
| HDC | 19.3 | 29.5 | 53.2 | 29.4 | 37.7 | 28.2 |
| PLDC | 19.0 | 23.6 | 24.2 | 21.9 | 22.7 | 3.9 |
| Changes in serum 1,25(OH)2D or serum paricalcitol, pg/mL | ||||||
| ERC | 29.0 | 32.5 | 12.4 | 50.4 | 54.1 | 7.2 |
| IRC | 27.2 | 39.7 | 46.3 | 32.1 | 43.7 | 36.0 |
| HDC | 23.9 | 36.7 | 53.1 | 30.2 | 34.7 | 14.8 |
| PLDC | ND | 19.9 | NC | 19.6 | 51.5 | 262.8 |
| Changes in serum 24,25(OH)2D₃, ng/mL | ||||||
| ERC | 0.923 | 0.923 | 0.0 | 2.878 | 2.953 | 2.6 |
| IRC | 0.991 | 1.168 | 17.8 | 1.085 | 1.307 | 20.5 |
| HDC | 0.954 | 1.008 | 5.6 | 1.264 | 1.349 | 6.8 |
| PLDC | 0.793 | 0.852 | 7.4 | 0.895 | 0.897 | 0.0 |
| ND = not detected | ||||||
| NC = not calculated | ||||||
Safety Parameters
No significant changes from baseline values were noted during treatment in any of the four groups for mean serum ionized calcium, total Ca, and P or for mean eGFR. However, an episode of hypercalcemia (confirmed serum Ca >10.3 mg/dL) was noted in one subject during PLDC treatment. Episodes of hyperphosphatemia (confirmed serum P >5.5 mg/dL) during treatment were observed once in the ERC group, twice in the PLDC group (once in each of two subjects), 3 times in the IRC group (all in a single subject), and eight times in the HDC group (multiple times in each of two subjects). One subject in the ERC group reduced the dosage from 60 to 30 μg/day and one in the PLDC group reduced the dosage of paricalcitol from 2 to 1 μg/day due to oversuppression of plasma iPTH (confirmed <30 pg/mL). At least one TEAE was observed in 39% of subjects in the ERC group, 39% in the IRC group, 44% in the HDC group, and 53% of the PLDC group (p < 0.05). None of the TEAEs were deemed related to study drug administration, and 94% were graded either mild or moderate. The most common TEAEs in all groups were gastrointestinal disorders (11–28%), primarily diarrhea and nausea, and nervous system disorders (11–20%), primarily headache and dizziness. Thirteen of the TEAEs occurring in 8 subjects were considered serious adverse events, one of which (rectal hemorrhage) occurred in a subject treated with ERC, four occurred in two subjects treated with IRC (one experienced melena, and the other acute kidney injury, diabetic ketoacidosis, and then fatal acute respiratory distress syndrome, possibly due to COVID-19), five occurred in four subjects treated with HDC (acalculous cholecystitis, blister, pulmonary edema, and quadrantanopia plus hypertensive encephalopathy), and three in a subject treated with PLDC (intervertebral disc protrusion, lumbar spinal stenosis, and lumbar radiculopathy).
Discussion
This prospective, open-label RCT evaluated iPTH-lowering responses after administration of different therapies capable of raising serum total 25OHD in relation to PLDC therapy in patients with SHPT, VDI, and stage 3 or 4 CKD. The data showed that daily ERC produced plasma iPTH-lowering response rates similar to those achieved with daily PLDC. In contrast, iPTH-lowering response rates achieved with IRC and HDC were significantly lower. ERC corrected VDI, defined as serum total 25OHD below 30 ng/mL, in 100% of treated subjects, whereas IRC, HDC, and PLDC were effective in only 20%, 44%, and 14% of subjects, respectively. Only ERC raised serum 25OHD to ≥50 ng/mL, achieving this target in 100% of subjects by the end of 2 months of treatment. The safety profile of ERC was favorable, characterized by fewer episodes of hypercalcemia, hyperphosphatemia, and serious adverse events than seen with IRC, HDC, or PLDC.
Data from the current study support and extend those reported previously from an open-label RCT conducted in the same population which compared single oral ERC doses of 450 or 900 μg to a single intravenous IRC dose of 448 μg [11]. In that earlier study, the 900 μg ERC dose produced greater and more sustained iPTH suppression compared to IRC despite a lower bioavailability (25 vs. 100%) and total 25OHD exposure. Mean percentage reductions in iPTH from baseline were minimal over the postdose period for both IRC and the lower ERC dose, but reached approximately 20% for the higher ERC dose (p < 0.05). The maximum concentration (Cmax) of serum total 25OHD was 134 ng/mL at 0.5 h for IRC, 25 ng/mL at 13.1 h for the lower ERC dose, and 33 ng/mL at 13.6 h for the higher ERC dose. Subsequent studies with ERC in predialysis patients have demonstrated that iPTH-lowering responses are directly proportional to the degree to which serum total 25OHD is elevated, with clinically meaningful responses occurring most frequently when levels gradually (not suddenly) surpass 50 ng/mL [12].
Single- and repeated-dose PK data on vitamin D metabolites from the current study show that serum total 25OHD elevation with ERC was far more gradual than with either IRC or HDC. Increases in mean serum total 25OHD after the first and 29th doses of ERC (on days 1 and 29) were fully physiological (1.4–2.4 ng/mL) within the ensuing 24-h postdose periods compared with more pharmacological changes observed with IRC (8.0–16.0 ng/mL) and HDC (8.0–10.2 ng/mL). The physiological changes in serum 25OHD with ERC minimized perturbations in serum 1,25(OH)2D (7.2–12.4%), whereas sudden, substantial increases in serum 1,25(OH)2D with IRC (36.1–46.0%) promoted increased expression of CYP24A1 in target tissues (including the parathyroid gland) and increased the intracellular catabolism of vitamin D metabolites [13], effectively reducing delivery of hormone to the vitamin D receptor and yielding lower iPTH responses. Postdose increases in serum 24,25(OH)2D3 with IRC were 18.2–20.0% and much lower with HDC (5.6–6.8%) and ERC (0.0–2.6%). The lack of large increases in serum 24,25(OH)2D3 with PLDC (0.0–7.4%) was likely due to compensatory decreases in serum 1,25(OH)2D. Limitations associated with bolus dosing regimens for immediate-release vitamin D therapies have been recently reviewed elsewhere [14].
Other studies in predialysis patients have shown that IRC failed to produce clinically meaningful reductions in PTH (>30% from pretreatment baseline) unless administered at doses that raised mean serum total 25OHD to levels approaching or exceeding 100 ng/mL, the upper limit of the laboratory normal range. In one study [15], subjects were treated with oral calcifediol (10–50 µg per day) or calcium carbonate (control) for 2 years. The PTH responses in the two treatment groups were reported as “comparable” and, in aggregate, PTH decreased by 4%. Another study [16] reported that a much higher oral dose of calcifediol (160 μg per day) reduced PTH by only 6%. A third study [17] reported a mean decrease in PTH of 21%, but only at oral doses of 200 μg/day or intravenous doses of 500 μg every 1–5 days. Although serum 25OHD levels in this third study were not reported, such high doses most certainly produced sustained levels far above 100 ng/mL. In a further study [18], 125 μg of oral calcifediol administered thrice weekly for 6 months yielded a mean PTH decline from 99.5 to 60.3 pg/mL (−39%) with serum 25OHD exceeding 100 ng/mL in nearly half of the subjects. Taken together, data from these previous studies and from the current study support the conclusion that gradual delivery of calcifediol allows more effective treatment of SHPT in CKD patients without excessively raising serum 25OHD.
Data from the current study also support conclusions that vitamin D supplementation with either cholecalciferol or ergocalciferol is ineffective for sufficiently elevating serum total 25OHD to effectively treat SHPT associated with VDI in CKD [4–6]. Only 44% of subjects treated with HDC achieved the serum 25OHD threshold of 30 ng/mL, currently endorsed by the K/DOQI and Endocrine Society Clinical Practice Guidelines [1, 19], and none achieved the more clinically relevant target of 50 ng/mL [12].
The findings from the current study contribute to an ongoing dialogue about the optimum level for serum total 25OHD in patients with stage 3 or 4 CKD, and how that level is best achieved. Recent studies [12, 20] suggest that this target should be at least 50 ng/mL and not 20 or 30 ng/mL as endorsed by clinical practice guidelines [1, 19] or based on data from healthy volunteers [9]. In the current study, most subjects (57%) who achieved a 30% reduction in plasma iPTH from pretreatment baseline had attained an EOT serum 25OHD level of ≥50 ng/mL with ERC. No increase in hypercalcemia, hyperphosphatemia, or other TEAEs was apparent with gradual elevation of serum 25OHD above this level in the ERC group, an observation which is consistent with previous findings [12, 21, 22] and conflicts with the expressed concerns of the Institute of Medicine [9] that elevation of serum 25OHD above 50 ng/mL may be harmful.
A factor in the inability of HDC to increase serum 25OHD to targeted levels (30 or 50 ng/mL) is excessive body weight: patients with CKD tend to be overweight or obese, a problem which drives disease progression through the comorbidities of type 2 diabetes and hypertension. The mean body weight of subjects in this trial was 102.2 kg and the mean BMI was 35.1 kg/m2, values which align well with those reported from previous prospective studies conducted in US patients with stage 3–4 CKD [21, 22]. Cholecalciferol and ergocalciferol are nonpolar molecules which have low affinities for the serum-based vitamin D binding protein. Consequently, they accumulate in adipose tissue [23, 24] where they are largely unavailable for hepatic conversion to 25OHD and prone to catabolism by CYP24A1, the vitamin D catabolic enzyme which is often upregulated in CKD patients [25]. Further, obesity has been reported to decrease hepatic 25-hydroxylase activity [26]. An inverse relationship has been consistently observed in the absence of CKD between serum 25OHD and both body weight and BMI [27–31]. Observations of this relationship have been recently extended to include patients with stage 3–5 CKD [32] and indicate that obesity limits the effectiveness of cholecalciferol and ergocalciferol to treat SHPT.
The current study provides part of a foundation for making changes in the traditional paradigm for treating SHPT in predialysis patients. According to this paradigm, patients are aggressively supplemented with cholecalciferol or ergocalciferol in order to correct VDI by raising serum total 25OHD to about 30 ng/mL and then are switched to therapy with calcitriol (or a 1α-hydroxylated vitamin D analog) when iPTH rises progressively above the normal range. Justification for this switch is the observed inability of these supplements to correct VDI and the concern that the administered supplements cannot be sufficiently activated to 1,25(OH)2D due to excessive loss of renal CYP27B1. Data from the current study support a better paradigm, namely that ERC should be initiated in lieu of supplements at an initial dose of 30 μg/day escalating, as needed, to 60 μg/day and that patients should not be so readily switched to calcitriol or 1α-hydroxylated vitamin D analog. Control of progressively rising iPTH should be addressed, instead, by safely raising serum 25OHD to sufficiently high levels (≥50 ng/mL) with ERC.
Certain elements of this study’s design and conduct may be subject to criticism. The choice of the dosing regimens studied may be challenged, as previously admitted (see Rationale for Selected Doses above). The duration of the treatment period may be criticized: it was 8 weeks, too short to allow high steady-state levels of serum 25OHD to be achieved with ERC after normal dose titration. Further, the study was insufficiently powered to statistically detect small differences in PD responses which may have existed between the ERC and the PLDC treatment groups; however, significant differences were detected between groups for the key parameters. Finally, premature termination of the study due to the onset of the COVID-19 pandemic may have introduced biases into the results which, under the circumstances, were unavoidable.
Conclusion
This 8-week prospective, randomized, controlled trial evaluated iPTH-lowering responses after administration of three different therapies capable of raising serum total 25OHD in comparison to a reference therapy (paricalcitol), which is incapable of raising serum 25OHD in patients with SHPT, VDI, and stage 3 or 4 CKD. The results showed that daily ERC treatment was safer and more effective at raising serum total 25OHD and decreasing elevated plasma iPTH than once monthly IRC or HDC. Plasma iPTH-lowering response rates achieved with daily ERC were similar to those seen with daily PLDC, demonstrating that ERC is an attractive alternative to widespread hormone replacement therapy in predialysis patients, which is now discouraged by the updated KDIGO clinical practice guideline for the treatment of CKD-MBD. Data from this study challenge the traditional paradigm for treating SHPT and underscore the urgency to adopt a better one that appropriately includes ERC.
Acknowledgments
The authors acknowledge the support of the following investigators and their staff in undertaking this study: Carlos Sanabria MD, Spaulding Clinical Research, LLC, West Bend, WI; Logan Elangovan MD, Nephrology Associates, Waukesha, WI; Paul Crawford MD, Research by Design, Chicago, IL; and Ahmed Awad, Clinical Research Consultants, Kansas City, MO.
Statement of Ethics
Subjects included in the clinical study gave written informed consent. The study protocol was approved by an Institutional Review Board (Advarra, Columbia, MD; approval number PRO00025210).
Conflict of Interest Statement
Three authors (S.A.S., A.A., C.W.B.) are employees of the Renal Division of OPKO Health, and one (PC) is an employee of Vifor Pharma.
Funding Sources
This study was supported by the Renal Division of OPKO Health and Vifor Pharma.
Author Contributions
All authors designed the study. S.A.S. carried out data analyses. S.A.S., P.C., A.A., and C.W.B. drafted and approved the manuscript.
Funding Statement
This study was supported by the Renal Division of OPKO Health and Vifor Pharma.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. National Kidney F. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- 2. Kidney Disease Improving Global Outcomes KDIGO CKD-MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009(113):S1–130. 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes KDIGO CKD-MBD Update Work Group . KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1):S1–S59. 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(9):1529–39. 10.2215/CJN.02140309. [DOI] [PubMed] [Google Scholar]
- 5. Agarwal R, Georgianos PI. Con: nutritional vitamin D replacement in chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant. 2016;31(5):706–13. 10.1093/ndt/gfw080. [DOI] [PubMed] [Google Scholar]
- 6. Bover J, Gunnarsson J, Csomor P, Kaiser E, Cianciolo G, Lauppe R. Impact of nutritional vitamin D supplementation on parathyroid hormone and 25-hydroxyvitamin D levels in non-dialysis chronic kidney disease: a meta-analysis. Clin Kidney J. 2021;14(10):2177–86. 10.1093/ckj/sfab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cozzolino M, Ketteler M. Evaluating extended-release calcifediol as a treatment option for chronic kidney disease-mineral and bone disorder (CKD-MBD). Expert Opin Pharmacother. 2019;20(17):2081–93. 10.1080/14656566.2019.1663826. [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Chronic Kidney Disease Epidemiology Collaboration: using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 9. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar J, Tran NTG, Schomberg J, Streja E, Kalantar-Zadeh K, Pahl M. Successful conversion from parenteral paricalcitol to pulse oral calcitriol for the management of secondary hyperparathyroidism in hemodialysis patients. J Ren Nutr. 2016;26(4):265–9. 10.1053/j.jrn.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petkovich M, Melnick J, White J, Tabash S, Strugnell S, Bishop CW. Modified-release oral calcifediol corrects vitamin D insufficiency with minimal CYP24A1 upregulation. J Steroid Biochem Mol Biol. 2015;148:283–9. 10.1016/j.jsbmb.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 12. Strugnell SA, Sprague SM, Ashfaq A, Petkovich M, Bishop CW. Rationale for raising current clinical practice guideline target for serum 25-hydroxyvitamin D in chronic kidney disease. Am J Nephrol. 2019;49(4):284–93. 10.1159/000499187. [DOI] [PubMed] [Google Scholar]
- 13. Petkovich M, Bishop CW. Extended-release calcifediol in renal disease. Health, Disease and Therapeutics. Vitamin D. 4th edEdition Elsevier; 2018. [Google Scholar]
- 14. Mazess RB, Bischoff-Ferrari HA, Dawson-Hughes B. Vitamin D: bolus is bogus-A narrative review. JBMR Plus. 2021;5(12):e10567. 10.1002/jbm4.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fournier A, Idrissi A, Sebert JL, Gueris J, Garabedian M, Renaud H, et al. Preventing renal bone disease in moderate renal failure with CaCO3 and 25(OH)-vitamin D3. Kidney Int Suppl. 1988;24:S178–179. [PubMed] [Google Scholar]
- 16. Letteri JM, Kleinman LM, Ellis KN, Caselnova R, Akhtar M, Cohn SH. Effects of 25-hydroxycholecalciferol on calcium metabolism in chronic renal failure. Adv Exp Med Biol. 1977;81:591–601. 10.1007/978-1-4613-4217-5_57. [DOI] [PubMed] [Google Scholar]
- 17. Bordier PJ, Marie PJ, Arnaud CD. Evolution of renal osteodystrophy: correlation of bone histomorphometry and serum mineral and immunoreactive parathyroid hormone values before and after treatment with calcium carbonate or 25-hydroxycholecalciferol. Kidney Int Suppl. 1975;2:102–12. [PubMed] [Google Scholar]
- 18. Levin A, Tang M, Perry T, Zalunardo N, Beaulieu M, Dubland JA, et al. Randomized controlled trial for the effect of vitamin D supplementation on vascular stiffness in CKD. Clin J Am Soc Nephrol. 2017;12(9):1447–60. 10.2215/CJN.10791016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 20. Ennis JL, Worcester EM, Coe FL, Sprague SM. Current recommended 25-hydroxyvitamin D targets for chronic kidney disease management may be too low. J Nephrol. 2016;29(1):63–70. 10.1007/s40620-015-0186-0. [DOI] [PubMed] [Google Scholar]
- 21. Sprague SM, Crawford PW, Melnick JZ, Strugnell SA, Ali S, Mangoo-Karim R, et al. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am J Nephrol. 2016;44:316–25. 10.1159/000450766. [DOI] [PubMed] [Google Scholar]
- 22. Sprague SM, Silva AL, Al-Saghir F, Damle R, Tabash SP, Petkovich M, et al. Modified-release calcifediol effectively controls secondary hyperparathyroidism associated with vitamin D insufficiency in chronic kidney disease. Am J Nephrol. 2014;40(6):535–45. 10.1159/000369939. [DOI] [PubMed] [Google Scholar]
- 23. Hengist A, Perkin O, Gonzalez JT, Betts JA, Hewison M, Manolopoulos KN, et al. Mobilising vitamin D from adipose tissue: the potential impact of exercise. Nutr Bull. 2019;44(1):25–35. 10.1111/nbu.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nimitphong H, Park E, Lee MJ. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr Res Pract. 2020;14(6):553–67. 10.4162/nrp.2020.14.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78(5):463–72. 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]
- 26. Roizen JD, Long C, Casella A, O'Lear L, Caplan I, Lai M, et al. Obesity decreases hepatic 25-hydroxylase activity causing low serum 25-hydroxyvitamin D. J Bone Miner Res. 2019;34(6):1068–73. 10.1002/jbmr.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Pergola G, Martino T, Zupo R, Caccavo D, Pecorella C, Paradiso S, et al. 25-Hydroxyvitamin D levels are negatively and independently associated with fat mass in a cohort of healthy overweight and obese subjects. Endocr Metab Immune Disord Drug Targets. 2019;19(6):838–44. 10.2174/1871530319666190122094039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rafiq S, Jeppesen PB. Body mass index, vitamin D, and type 2 diabetes: a systematic review and meta-analysis. Nutrients. 2018;10(9):1182. 10.3390/nu10091182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samuel L, Borrell LN. The effect of body mass index on optimal vitamin D status in U.S. adults. The National Health and Nutrition Examination Survey 2001-2006. Ann Epidemiol. 2013;23(7):409–14. 10.1016/j.annepidem.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 30. Saneei P, Salehi-Abargouei A, Esmaillzadeh A. Serum 25-hydroxyvitamin D levels in relation to body mass index: a systematic review and meta-analysis. Obes Rev. 2013;14(5):393–404. 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
- 31. Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25-hydroxyvitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. 2008;27(2):274–9. 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bishop CW, Strugnell SA, Csomor P, Kaiser E, Ashfaq A. Extended-release calcifediol effectively raises serum total 25-hydroxyvitamin D even in overweight nondialysis chronic kidney diesease patients with secondary hyperparathyroidism. Am J Nephrol. 2022:53(6):446–54. 10.1159/000524289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.