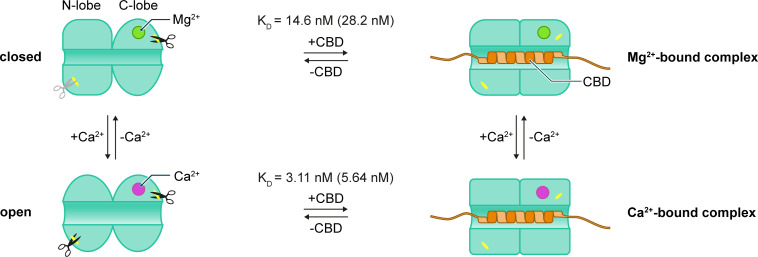
Figure 7. Conformation of CHP3 is controlled by Ca2+ and target peptide (NHE1 CBD) binding.
Ca2+ (magenta) replaces Mg2+ (green) in EF-3 and induces the transition from the closed to the open conformation. Ca2+ binding affects the local flexibility in the N-lobe accelerating the tryptic cleavage and reduces the thermal stability of CHP3. In both, Ca2+- and Mg2+-bound states, CHP3 binds the target peptide CBD with nanomolar affinity (KD values indicated, in brackets for myristoylated CHP3). Complex formation has no effect on the thermal stability of the Mg2+-bound state (closed conformation), whereas it strongly enhances thermal stability of the Ca2+-bound state (open conformation). Independent of the bound ion, both trypsin cleavage sites are protected from proteolysis in the CHP3:CBD complex.