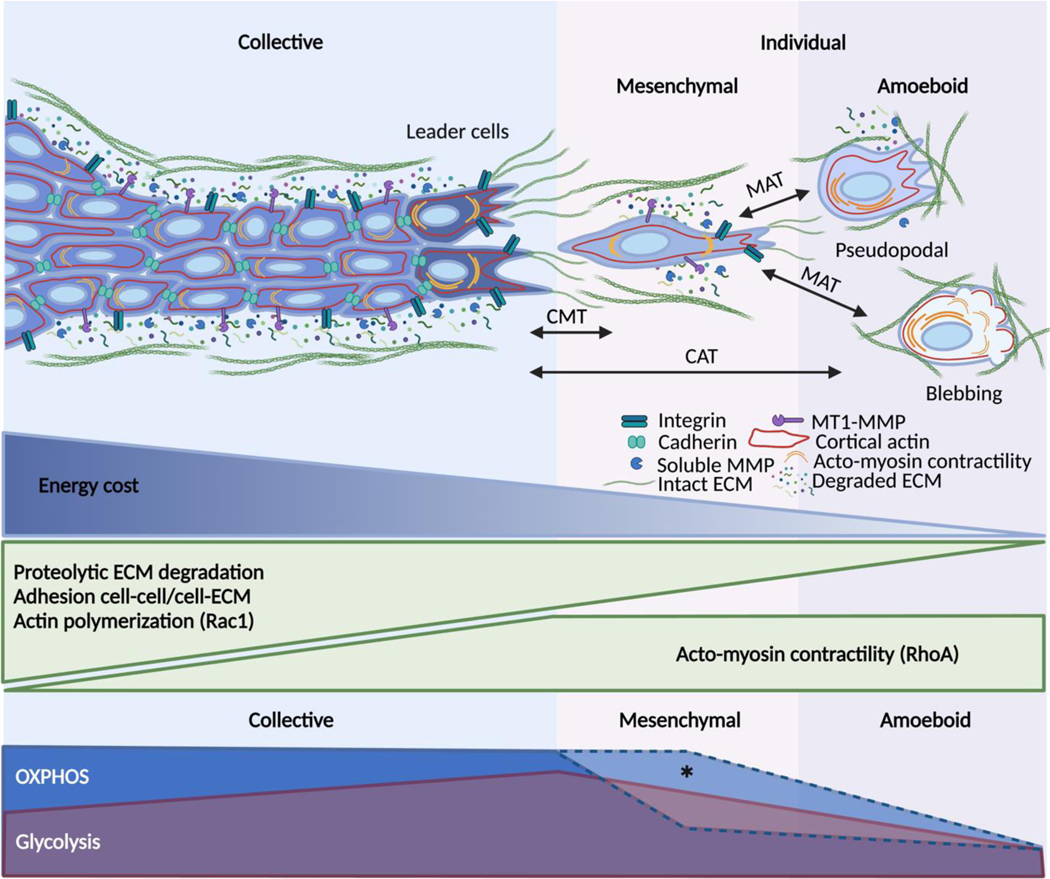
Fig. 2. Interdependence of energy consumption and migration strategy.
Collective migration depends on strong cell-cell adhesion, Rac1-mediated actin dynamics, Rho-A mediated contractility, integrin-mediated ECM adhesion and deformation together with pericellular proteolysis. Because of its molecular and mechanical complexity, collective migration is energetically costly, particularly for the leader cells that must overcome substrate resistance. Collective-to-mesenchymal (CMT) single-cell transition is mediated by the downregulation of intercellular adhesions. Losing cell-cell junctions allows mesenchymal single cells to save some energy, even though their elongated morphology still requires actin activity at the leading edge, cytoskeletal contractility, ECM-adhesion, and proteolysis. Mesenchymal-to-amoeboid transition (MAT) results from lowering adhesion to the substrate and pericellular proteolysis. The pseudopodal amoeboid mode retains actin-rich protrusions while the blebbing mode completely relies on Rho-mediated actomyosin contractility. By lowering most of the ATP-consuming steps of motility, the amoeboid mode seems to minimize the energy demands of migration. The lower panel shows the hypothetical coupling of migration modes and metabolic reprogramming. Created with BioRender.com