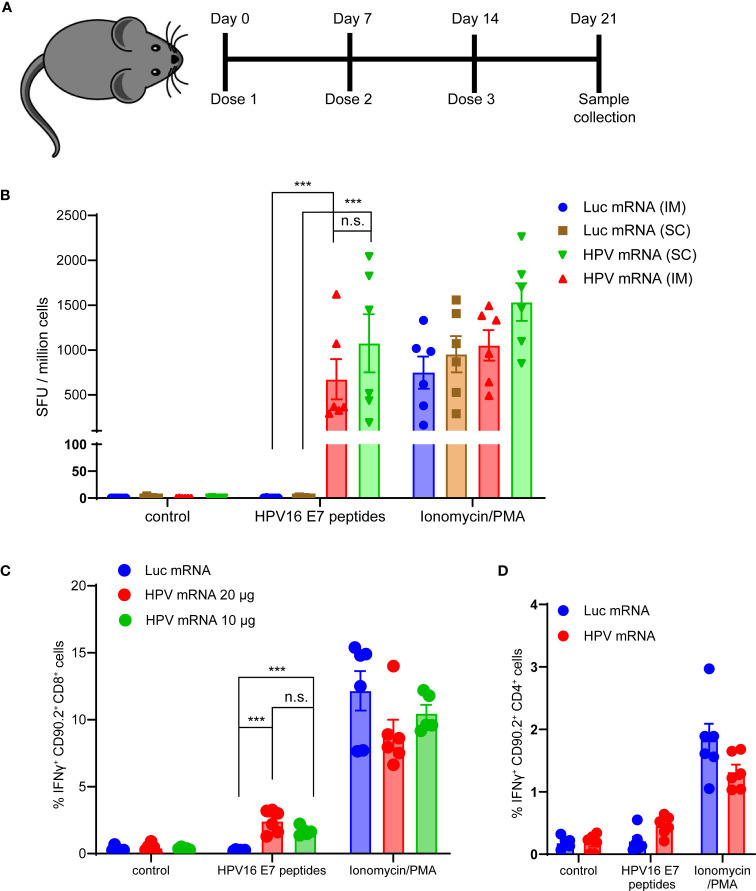
Figure 3.
(A) Schema of the study design. (B) ELISpot analysis of IFNγ positive Spot forming units (SFU) in splenocytes derived from mice vaccinated with 10 μg of MC3-based LNP-formulated mRNA vaccine, given either via intramuscular (im) or subcutaneous (sc) injection, and stimulated ex vivo with either a vehicle control, 5 μg/mL HPV16 E7 peptides or Ionomycin/PMA control. Data presented as mean ± SEM. Differences between groups were tested using two-way ANOVA test (***, P<0.001; n.s., no significance). (C) Flow Cytometry analysis of IFNγ positive Cytotoxic T lymphocytes from splenocytes derived from mice vaccinated with either 10 or 20 μg of MC3-based LNP-formulated mRNA vaccine, or with 20 μg of MC3-based LNP-formulated luciferase mRNA control, and stimulated ex vivo with either a vehicle control, 5 μg/mL HPV16 E7 peptides or Ionomycin/PMA control. Data presented as mean ± SEM. Differences between groups were tested using two-way ANOVA test (***, P<0.001; n.s., no significance). (D) Flow Cytometry analysis of IFNγ positive Helper T lymphocytes from splenocytes derived from mice vaccinated with either 20 μg of MC3-based LNP-formulated mRNA vaccine, or with 20 μg of MC3-based LNP-formulated luciferase mRNA control, and stimulated ex vivo with either a vehicle control, 5 μg/mL HPV16 E7 peptides or Ionomycin/PMA control. Data presented as mean ± SEM. Differences between groups were tested using two-way ANOVA test (n.s., no significance).