Figure 6.
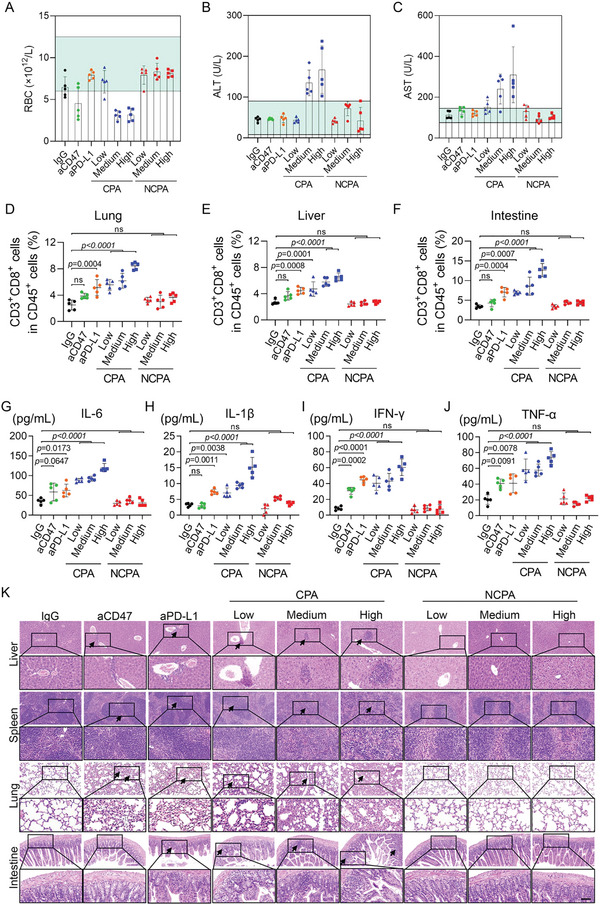
NCPA reduced the incidence of IRAEs in subcutaneous graft Lewis lung carcinoma‐bearing mice. A) Routine blood test results of RBCs (red blood cells) of mice on day 15 after the indicated treatments. (B&C) Blood biochemical analyses of liver function markers. B) ALT (alanine aminotransferase) and C) AST (aspartate aminotransferase) in the serum of mice on day 15 after the indicated treatments. D–F) Flow cytometry analysis of activated CD8+ T cell infiltration in D) lung, E) liver, and F) intestine on day 15 after the indicated treatments. G–J) ELISA analysis of inflammatory cytokines in serum, including G) IL‐6, H) IL‐1β, I) IFN‐γ, and J)TNF‐α. K) Ex vivo pathological H&E staining of the liver, spleen, lung, and intestine of mice receiving indicated treatments on day 15. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
