Figure 4.
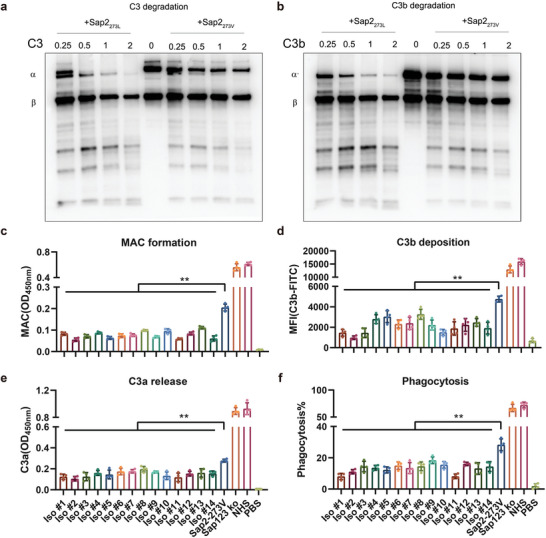
Site mutated Sap2273L, by potentially degrading C3 and C3b, displayed stronger complement inhibitory effects. a,b) Sap2 mediated concentration‐dependent degradation of C3/C3b. Different doses (0.25, 0.5, 1, 2 µg) of purified Sap2273V and Sap2273L proteins were incubated with purified human C3 or C3b (0.5 µg), afterward, the mixtures were separated by SDS‐PAGE under reducing conditions, and the degradation products of C3 (a) and C3b (b) were detected by western blotting using polyclonal goat anti human C3 antibody. c) Effect of V273L variation of Sap2 on MAC formation, PBS indicated a negative background control without NHS in the sample. d) Effect of V273L variation of Sap2 on C3b/iC3b deposition, PBS indicated a negative background control without NHS in the sample. e) Effect of V273L variation of Sap2 on C3a release, PBS indicated a negative background control without NHS in the sample. f) Effect of V273L variation of Sap2 on C3b/iC3b mediated phagocytosis of C. albicans by THP‐1. The phagocytosis of Sap2‐273V strain by THP‐1 cells was quantified by flow cytometry as double positive cells (FITC+, DiD+), PBS indicated a negative background control without C. albicans. Data are shown as means ± SD, and from one of three independent experiments. p values were analyzed by Student's t‐test, **p < 0.01.
