Figure 6.
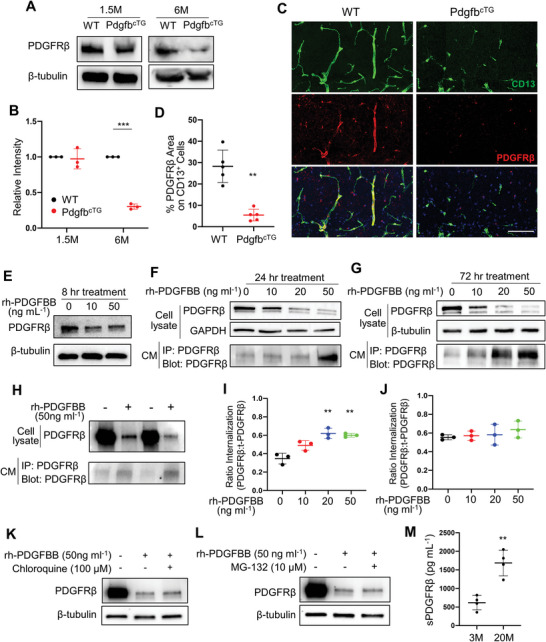
Persistent exposure to high concentrations of PDGF‐BB ligand leads to its receptor shedding in pericytes. A) PDGFRβ protein expression in hippocampal tissue collected from 1.5‐ and 6‐month‐old PdgfbcTG mice and WT littermates was assessed by Western blot analysis. B) Quantification of the relative intensity of PDGFRβ by Image J. n = 3. C) Representative confocal images of CD13 (green) and PDGFRβ (red) double‐immunofluorescence staining in CA1 region of 6‐month‐old PdgfbcTG mice and WT littermates. DAPI stains nuclei as blue. Scale bar, 100 µm. D) Quantification of the percentage of PDGFRβ area on CD13+ pericytes in hippocampus. n = 5. E–G) Primary human brain pericytes were treated with different dosages of recombinant human PDGF‐BB (rh‐PDGFBB) for 8 (E), 24 (F), and 72 h (G). PDGFRβ protein expression was detected by Western blot analysis. H) Primary human brain pericytes were treated with rh‐PDGFBB for 24 h, and culture medium (CM) was collected. PDGFRβ expression in cell lysate was detected by Western blot analysis (upper panel), and the cleaved sPDGFRβ in CM was immunoprecipitated by a specific PDGFRβ antibody followed by Western blot analysis of PDGFRβ expression (lower panel). I,J) Primary human brain pericytes were treated with rh‐PDGFBB for 24 (I) and 72 h (J), and intracellular ratio was measured by flow cytometry. K,L) Primary human brain pericytes were treated with rh‐PDGFBB together with Chloroquine (K) or MG‐132 (L) for 72 h. PDGFRβ protein expression was detected by Western blot analysis. n = 3. M) ELISA analysis of CSF sPDGFRβ concentrations in C57BL/6 mice at 3 and 22 months of age. n = 4. Data are shown as the mean ± SD, **p<0.01, ***p<0.001, unpaired two‐tailed Student's t test.
