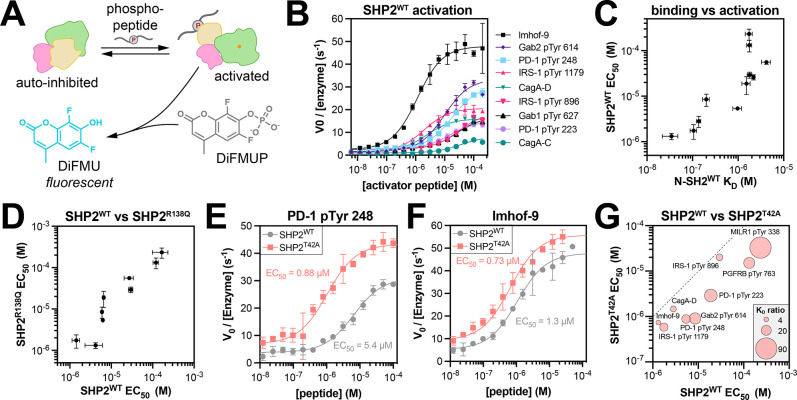
Figure 6. T42A-dependent changes in the activation of full-length SHP2.
(A) SHP2 activation is measured by incubation with phosphopeptide ligands, followed by monitoring dephosphorylation of the small-molecule substrate DiFMUP to generate fluorescent DiFMU. (B) Representative activation curves for SHP2WT, highlighting peptide-dependent changes in EC50 and amplitude. (C) Correlation between the EC50 of SHP2WT activation by phosphopeptides and the of those phosphopeptides for the N-SH2WT domain. (D) Correlation between activation EC50 values for SHP2WT and SHP2R138Q, which has weakened C-SH2 binding capacity. (E) Comparison of SHP2WT and SHP2T42A activation curves for the PD-1 pTyr 248 peptide, highlighting a significant impact on both EC50 and amplitude. (F) Comparison of SHP2WT and SHP2T42A activation curves for the Imhof-9 peptide, highlighting a minor change in EC50 and amplitude. (G) Bubble plot juxtaposing the EC50 values for activation of SHP2WT and SHP2T42A by nine peptides, alongside the fold-change in for binding of those peptides to N-SH2WT vs N-SH2T42A. The dotted line indicates where EC50 values would be equivalent for SHP2WT and SHP2T42A. The graph shows that peptides with a large fold-change in binding affinity (larger bubble) have a large fold-change in EC50 values for SHP2T42A over SHP2WT (distance from dotted line). All EC50 values can be found in Table S5.