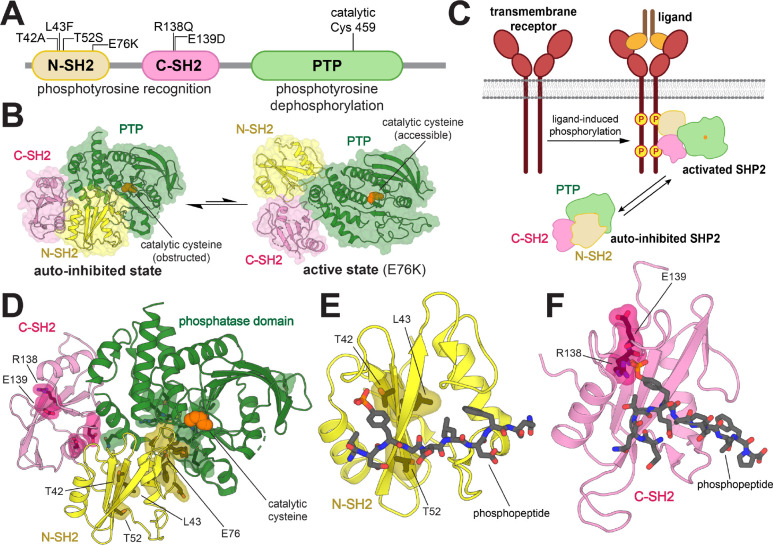
Figure 1. Structure and regulation of SHP2.
(A) Domain architecture diagram of SHP2. Relevant mutations and the catalytic cysteine (C459) are indicated. (B) SHP2 is kept in its auto-inhibited state by interactions between the N-SH2 and PTP domain (PDB: 4DGP). In its active state, the catalytic cysteine is accessible. The structure of SHP2E76K (PDB: 6CRF) is used to represent the active state. (C) The SH2 domains of SHP2 bind to tyrosine-phosphorylated upstream proteins, such as transmembrane receptors, inducing a conformational change that activates SHP2. (D) Disease-associated mutations cluster largely, but not exclusively, on the interdomain interface between the N-SH2 and the PTP domain (PDB: 4DGP). (E) Mutations in or near the N-SH2 binding pocket (PDB: 6ROY). (F) Mutations in or near the C-SH2 binding pocket (PDB: 6R5G).