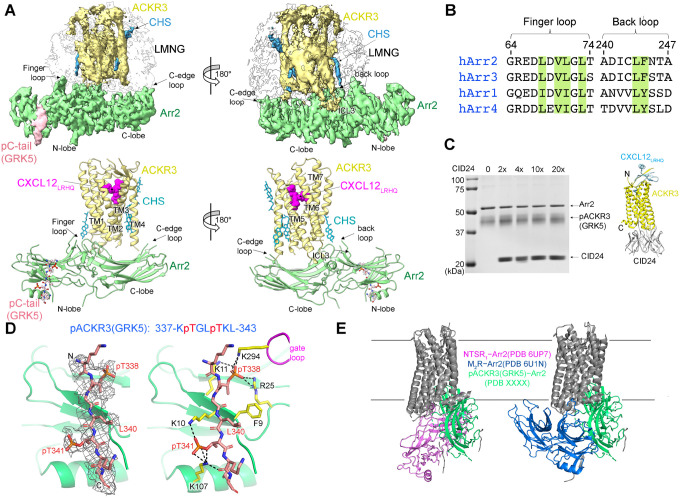
Figure 3. The interface between pACKR3(GRK5) and Arr2 is supported by a novel and several conventional interactions.
(A) Sharpened maps and models of pACKR3(GRK5)–Arr2 from the pACKR3(GRK5)–Arr2–Fab7 complex (PDB entry XXXX) with Fab7 density omitted. (B) Sequence alignment of the arrestin finger and back loops. Conserved hydrophobic residues involved in receptor and detergent/membrane binding are highlighted in green. (C) A flag pulldown assay in the absence or presence of increasing concentrations of CID24 shows no competition with Arr2 binding. CID24 binds to the cytoplasmic cleft of ACKR3 (PDB entry 7SK6) and thus blocks the access to the TM core. (D) Interactions of the pACKR3(GRK5) C-tail with the Arr2 N-lobe in the pACKR3(GRK5)–Arr2–Fab7 complex (PDB entry XXXX). Electron density for the pACKR3(GRK5) phospho-peptide is shown as a wire cage contoured at 12σ. Phosphate interactions below 4 Å are shown as black dash lines. (E) Comparison of Arr2 from the pACKR3(GRK5) structure with the NTSR1 (PDB entry 6UP7) and the M2R (PDB entry 6U1N) complexes after alignment of the receptor TM cores.