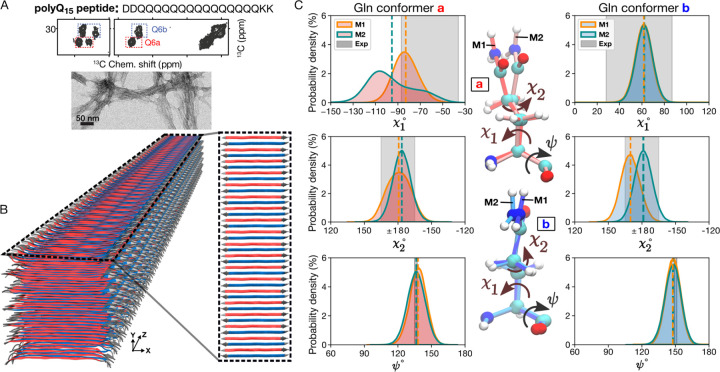
Figure 3. PolyQ peptide fibril structure.
(A) Amino acid sequence of D2Q15K2 peptide (top), 2D 13C–13C ssNMR spectrum (middle) of D2Q15K2 fibrils with single 13C-labeled Gln (Q6), and negatively-stained TEM of the peptide aggregates (bottom). The ssNMR data adapted from Ref. 8. Signals from type ”a” and ”b” Gln conformers are shown in red and blue boxes, respectively. (B) 3D cartoon representation of the structural model of the D2Q15K2 fibril. The alternation of β-strands of ”a” (red) and ”b” (blue) Gln conformers within a single sheet is shown on the right. (C) The χ1, χ2, and ψ dihedral angle distributions of “a” (left) and “b” (right) conformers in the context of the D2Q15K2 fibril for the M1 (orange) and M2 (green) models; dashed vertical lines represent mean values. The data were obtained from 1-μs MD simulations (using the OPLSAA/M46 force field; for Amber14SB45 see Supplementary Fig. S10). Gray shading depicts the ssNMR constraints for the dihedral angles. The structures at the center show representative “a” (top) and “b” (bottom) conformers of the M1 and M2 models.