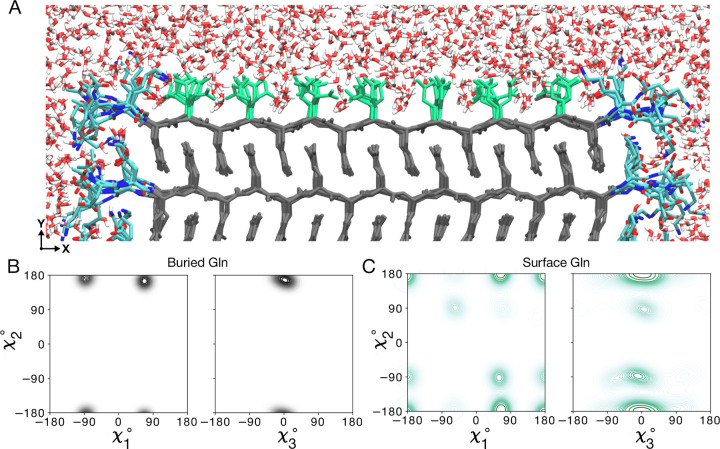
Figure 4. Atomic model of the water-facing surface of polyQ amyloid.
(A) Atomistic MD snapshot of the D2Q15K2 peptide fibril’s polyQ surface in contact with water. Exposed and buried Gln residues are colored green and gray, respectively. Note how the Gln side-chains internal to the (model M1, for M2 see Supplementary Fig. S12) amyloid core are well-ordered, while the water-facing side-chains display more mobility. (B) Side-chain dihedral angle distributions for the buried Gln residues and (C) for the Gln residues on the fibril surface (Amber14SB45; for OPLSAA/M46 see Supplementary Figs. S13, S14). The surface-facing residues show more disorder, but are nonetheless constrained to just few varyingly prominent specific rotamer states.