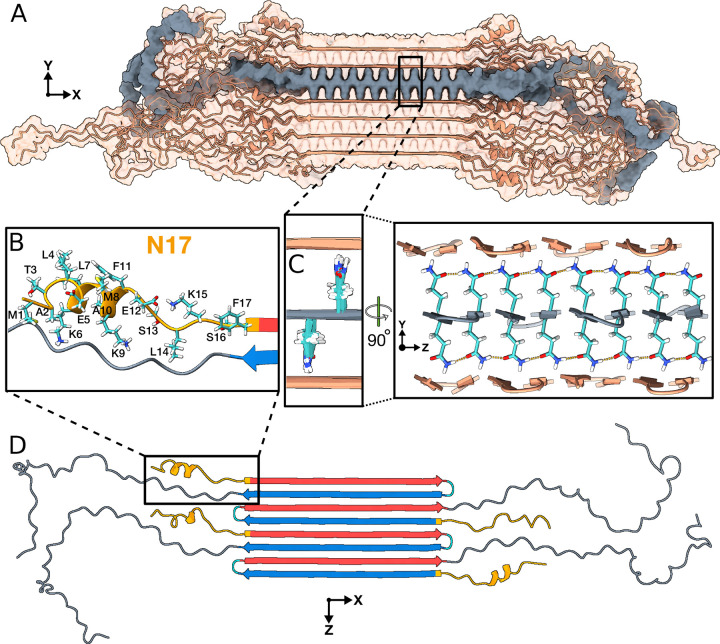
Figure 7. Structural analysis of the Q44-HTTex1 amyloid fibril.
For an illustrative 3D exploration of Q44-HTTex1 fibril structure, see the associated video in Extended Data 1. (A) A graphical depiction (after 5-μs simulation in Amber14SB45) of the HTTex1 fibril. The region shaded in gray denotes a single sheet within the fibril’s architecture. (B) An atomic view of the N17 domain within the fibril, naming the specific amino acids. (C) An atomic depiction of the glutamine side-chains within the fibril. The high stability of the fibril structure is primarily attributed to the extensive hydrogen bonding interactions among the glutamines, as depicted in the right panel. (D) Top view representation of the β-sheet highlighted in panel A. A quartet of HTTex1 monomers is visible. The polyQ is color-coded for the type “a” (red) and “b” (blue) strands; the tight β-turn is cyan. The N17 and PRD domains are orange and gray, respectively. Note the structural variation between different monomers in the same fibril sheet, including in particular the range of helical content in the N17.