Fig. 5. ACE increases neutrophil LTB4 production.
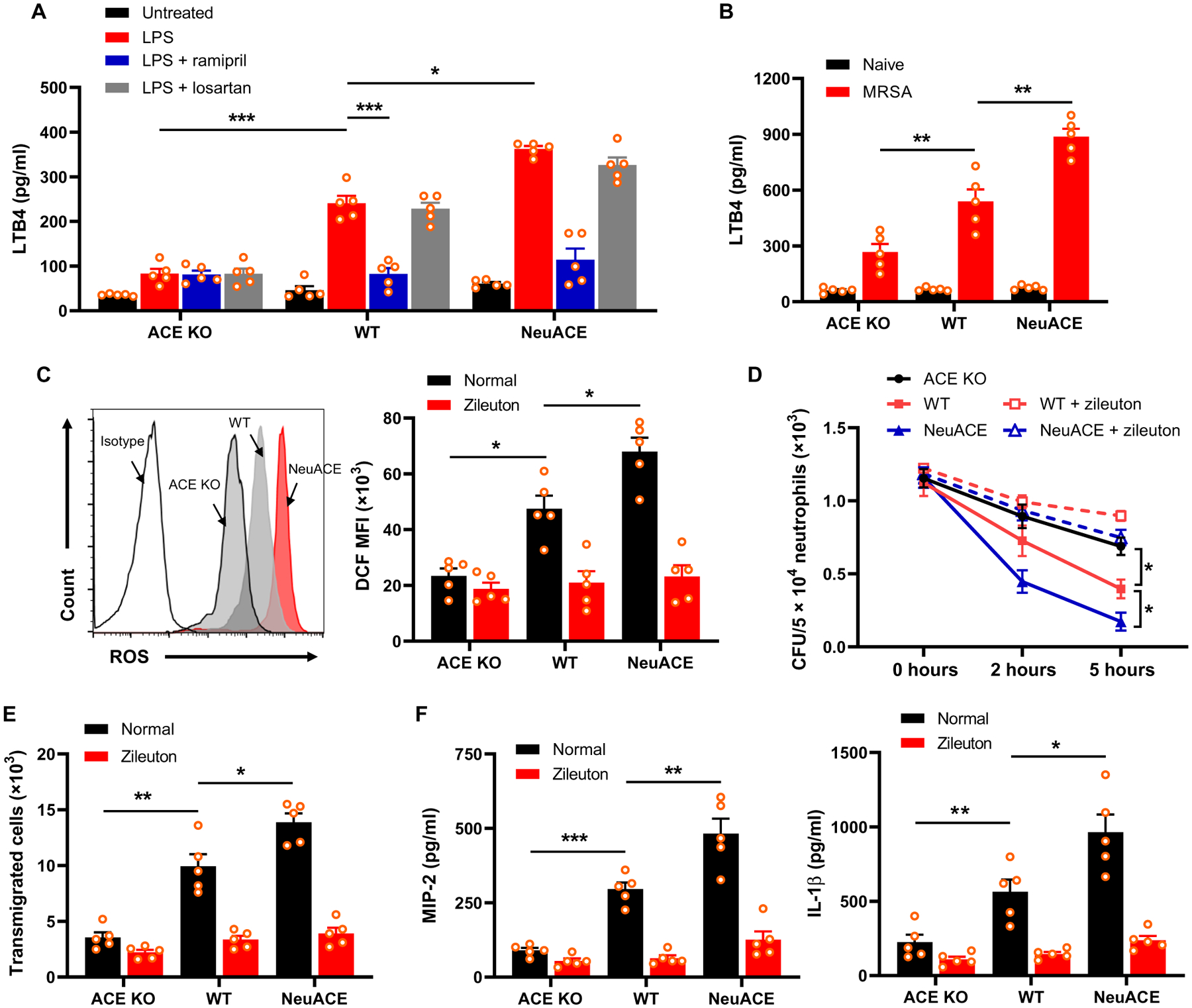
(A) Bone marrow neutrophils were cultured and stimulated with LPS (500 ng/ml) for 12 hours, and then, the supernatant plus cells were collected for ELISA. Groups of mice were treated with either ramipril (40 mg/liter) or losartan (600 mg/liter) in drinking water for 1 week before isolation of bone marrow neutrophils, and then, cells were treated with 10 μM ramipril or 100 μM losartan during the in vitro assay. (B) Measurement of LTB4 in serum isolated 24 hours after challenge with intravenous MRSA (1.2 × 108 CFU/100 μl). (C) Measurement of ROS production in neutrophils treated with LPS (1 μg/ml) for 30 min using 2’,7’-dichlorodihydrofluorescein diacetate (DCFDA) staining and flow cytometry. A representative flow cytometry plot is shown on the left, and quantification of mean fluorescence intensity (MFI) of ROS indicator 2’,7’-dichlorodihydrofluorescein (DCF) is shown on the right. To inhibit LTB4 synthesis, groups of mice were treated with the 5-LOX inhibitor zileuton (5 mg/kg per day, gavage) for 2 days before isolation of bone marrow neutrophils, and the drug was maintained at a concentration of 100 μM during in vitro assays. (D) Mice were pretreated with zileuton, and bone marrow–isolated neutrophils were challenged in vitro with MRSA at MOI 13 to evaluate intracellular killing. (E) Neutrophils were preincubated with 100 μM zileuton and then placed in the top chamber of a transwell to measure chemotaxis. The cells that migrated to the bottom chamber in response to 100 nM fMLP were collected and enumerated by cell counter. (F) Bone marrow neutrophils were cultured and stimulated with 100 nM fMLP for 12 hours. Supernatant along with cells were collected for ELISA to measure MIP-2 and IL-1β concentrations. Two-way ANOVA with Bonferroni’s correction for multiple comparisons was used to analyze group comparisons, and data are presented as means ± SEM (n = 5 per group). *P < 0.05, **P < 0.01, and ***P < 0.001.
