Abstract
Objective
We describe a male patient with covert sustained cognitive impairment who underwent endovascular treatment for severe stenosis in the left intracranial internal carotid artery (ICA).
Case Presentation
A 64-year-old man presented with transient dysarthria and dysphagia. Although he was alert, a cognitive evaluation revealed significant dysgraphia and a remarkable reduction in cognitive function. Diffusion-weighted imaging (DWI) revealed scattered high-intensity regions in the watershed area of the left cerebral hemisphere and severe stenosis in the C2 portion of the left ICA. Percutaneous transluminal angioplasty (PTA) was performed; a detailed examination revealed significantly improved cognitive function. One year later, the patient demonstrated further cognitive improvement, without any recurrent stroke.
Conclusions
We consider that patients with severe intracranial stenosis, who have covert cognitive decline without apparent sustained symptoms, might be promising candidates for revascularization. Higher brain function in patients with severe intracranial arterial stenosis should be carefully screened because cognitive decline might not be evident at the time of initial presentation.
Keywords: intracranial internal carotid artery stenosis, percutaneous transluminal angioplasty, revascularization therapy, cognitive impairment
Introduction
Intracranial internal carotid artery (ICA) stenosis, which is a relatively common occurrence among Asian populations including Japanese, sometimes causes cognitive decline in patients with or without evidence of ipsilateral ischemic lesions.1–7) Although cognitive function after carotid artery endarterectomy (CEA) or carotid artery stenting (CAS) improves in patients with extracranial ICA stenosis, the effects of percutaneous transluminal angioplasty (PTA) for cognitive impairment in patients with intracranial ICA stenosis have not been determined 3, 8, 9) Here, we describe a patient who underwent neuroendovascular treatment for cognitive impairment during the acute phase.
Case Report
A 64-year-old man presented with transient dysarthria and dysphagia. His medical history included hypertension, type 2 diabetes mellitus, and a duodenal ulcer. His family history was unremarkable. His social history included distilled alcoholic spirits, 360 mL/day and he smoked 20 cigarettes/day × 35 years.
The patient found that his right ring and little fingers had become numb 3 months before admission, then transient dysarthria for 5 minutes developed 2 months later. At 5 days before admission, he leaned somewhat to the right while walking. On the day of admission, he had transient right upper limb weakness and presented at our emergency department.
His consciousness score evaluated using the National Institutes of Health Stroke Scale (NIHSS) was 0, and he did not appear to have right hemiparesis or a gait disturbance. Electrocardiography revealed normal sinus rhythm, and blood tests found dyslipidemia and hyperuricemia. Diffusion-weighted imaging (DWI) showed scattered regions of high intensity in the watershed area of the left cerebral hemisphere, fluid-attenuated inversion recovery (FLAIR) imaging showed an intra-arterial sign in the middle cerebral artery area of the left cerebral hemisphere (Fig. 1A and 1B). Magnetic resonance angiography (MRA) confirmed the severe stenosis in the left ICA (Fig. 1C). The patient was medicated with edaravone 60 mg/day and aspirin 100 mg/day.
Fig. 1. MRI findings. Image shows asymptomatic, small, acute, ischemic stroke lesions (A), intra-arterial sign (B), and severe stenosis, in C2 portion of left ICA (C). ICA: internal carotid artery; MRI: magnetic resonance imaging.
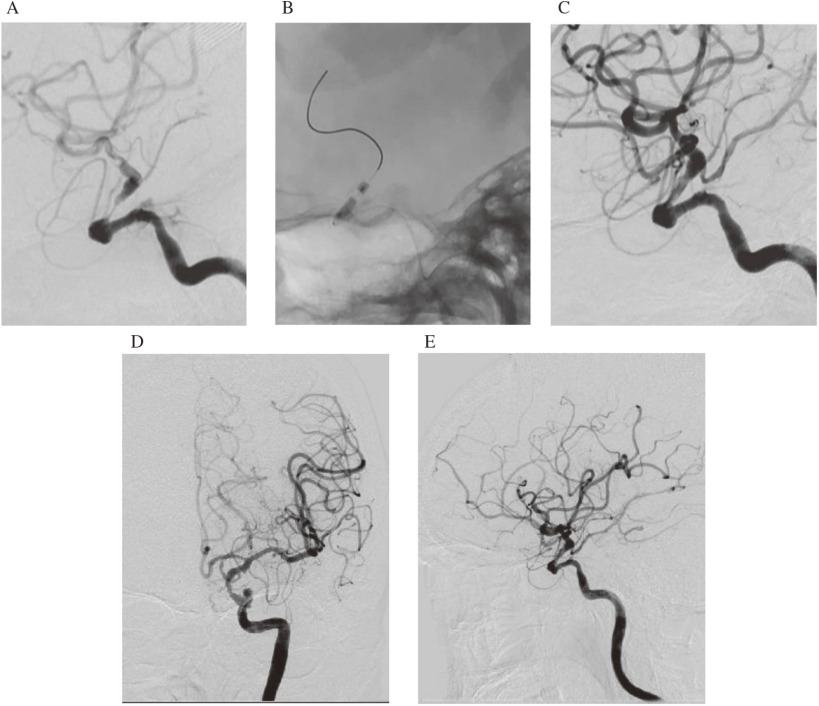
Cerebral arteriography on hospital day 3 showed 89% stenosis according to the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) assessment, stenosis of Mori A in the C2 portion of the left ICA, and poor collateral circulation from the posterior communicating artery (Pcom) (Fig. 2A–2D). Aspirin was increased to 200 mg/day, and clopidogrel 75 mg/day was added. Cerebral blood flow on quantitative N-isopropyl-p-123 iodoamphetamine single-photon emission computed tomography (SPECT) on hospital day 6 was significantly reduced in the left cerebral hemisphere (Fig. 3A–3C). In addition, evaluation scores between hospital days 2–8 showed significant dysgraphia and remarkably reduced higher brain function (Revised Hasegawa Dementia Scale (HDS-R), 16/30; Mini-Mental State Examination (MMSE), 21/30; frontal assessment battery (FAB), 10/18; Raven colored progressive matrices (RCPM), 21/36 and the Koh Block Design Test (KBDT), Koh IQ = 64.5. The clinical course during the previous months and the present status of the patient suggested risk for exacerbation. We judged that revascularization was necessary after exhaustive discussions including neurosurgeons and neurologists. Although evidence has yet to confirm that bypass surgery and endovascular treatment during the acute phase are effective, we selected the latter with reference to the patient and the preferences of his family. PTA proceeded on hospital day 14.
Fig. 2. Angiography findings. Frontal and lateral views show extreme stenosis of C2 portion in left ICA (A and B). Frontal view shows blood flow across anterior communicating artery to contralateral side (C) and little development of posterior communicating artery (D). ICA: internal carotid artery.
Fig. 3. Preoperative quantitative findings of N-isopropyl-p-123 iodoamphetamine SPECT findings. (A) Lateral ventricular body level, (B) basal ganglia level, and (C) cerebellum slice level. Blood flow in left cerebral hemisphere is significantly decreased. SPECT: single-photon emission computed tomography.
A 9-Fr Optimo balloon guiding catheter (Tokai Medical Products Inc., Kasugai, Aichi, Japan) was placed in the left ICA through the right femoral artery under local anesthesia. An intermediate 6Fr Cerulean guiding catheter (Medikit Co. Ltd., Tokyo, Japan) was placed in the ICA within the petrous temporal bone. A 0.014″ × 300-cm wire (Asahi Chikai 14; Asahi Intecc, Nagoya, Japan) was used with a 2.5 × 9 mm Gateway, over-the-wire PTA dilator microcatheter (Stryker, Tokyo, Japan). The Chikai 14 wire served as a road map to cross the wire into the side of the lesion. Thereafter, a 2.5 × 9 mm Gateway balloon catheter was guided over the wire into the lesion, then inflated twice to 6 atmospheres for 2 minutes to achieve an appropriate adequate vessel diameter of the target lesion. Progress was monitored because of suspected focal arterial dissection, but worsening did not occur and antegrade blood flow had apparently improved. The procedure was then completed (Fig. 4A–4E).
Fig. 4. Imaging findings of enlarged C2 portion lesion. PTA was followed by angiography (lateral view). Slight vascular dissection is evident (A–C). Frontal and lateral views of final angiogram show improved left ICA stenosis and cerebral flow (D and E). ICA: internal carotid artery; PTA: percutaneous transluminal angioplasty.
Clopidogrel 75 mg/day and aspirin 100 mg/day were continued postoperatively. Brain magnetic resonance imaging (MRI) on the day after surgery revealed an increase in asymptomatic cerebral infarction lesions, but the intra-arterial signals on FLAIR had almost completely disappeared (Fig. 5A and 5B). The stenosis had disappeared and the left ICA system was clearly visualized on MRA images (Fig. 5C). Cerebral blood flow evaluated by SPECT on postoperative day (POD) 7 showed improvement and no laterality in the left hemisphere (Fig. 6A–6C). Despite mild persistent dysgraphia on POD 2–8, scores for all measures were significantly improved: HDS-R, 23/30; MMSE, 22/30; FAB, 14/18; RCPM, 34/36; Koh IQ, 95.8. The patient was transferred to a rehabilitation hospital to convalesce because further symptomatic improvement was likely. No restenosis of the left ICA was evident on POD 387, and his cognitive function markedly improved in outpatient clinic according to the following scores: MMSE, 28/30; FAB, 15/18; and RCPM, 36/36.
Fig. 5. MRI findings. Asymptomatic acute ischemic stroke has slightly increased (A), intra-arterial sign has almost completely disappeared (B), stenosis has disappeared and left ICA system is clearly visualized (C). ICA: internal carotid artery; MRI: magnetic resonance imaging.
Fig. 6. Postoperative quantitative N-isopropyl-p-123 iodoamphetamine SPECT findings. (A) Lateral ventricular body level, (B) basal ganglia level, and (C) cerebellum slice level. Blood flow is normalized in left cerebral hemisphere. SPECT: single-photon emission computed tomography.
Discussion
Both cerebral blood flow and cognitive function were considerably improved by PTA in a patient with intracranial ICA stenosis. Among Asians with atherothrombotic stroke, 25% have intracranial artery stenosis or occlusion.10) In addition, the rate of atherothrombosis recurrence in an intracranial major artery during medical therapy is especially high in such patients.11) However, the effects of cerebral endovascular treatment in patients with intracranial stenosis have not been obvious, and most previous large trials have not achieved favorable outcomes.12–14)
Recent studies of cognitive function and intracranial atherosclerosis have found a relationship between posterior cerebral artery plaque and mild cognitive impairment, and a positive relationship between dementia, anterior cerebral artery plaque, ≥2 areas of plaques, and ≥50% stenosis.1) Although all these findings were derived from Caucasians, ≥50% stenosis in the intracranial arteries is considered to lead to dementia and cognitive impairment, and affect higher brain function.2)
Many investigators have found that intracranial or extracranial arterial stenosis can affect cognitive function or mood disturbance. According to WASID evaluation, MMSE scores are significantly lower in patients with >70% than 40–70% ICA stenosis.3) Pucite et al. reported that the quality of life is reduced when severe intracranial stenosis is complicated by peripheral artery disease.4) Everts et al. found a significant relationship between >70% stenosis and anxiety.5) Lal et al. investigated patients with asymptomatic, severe ICA stenosis using transcranial Doppler ultrasonography and found a positive relationship between the breath-holding index and cognitive function, suggesting a relationship between hemodynamic components and cognitive dysfunction.6) Another study showed that cerebral infarction can impair cognitive function even in patients with asymptomatic ICA stenosis, thus suggesting the importance of early intervention.7)
The effects of CEA and CAS on cerebral blood flow have been demonstrated, and their possible effects on cognitive function have been determined using the Montreal Cognitive Assessment (MoCA).15) A study of Cognistat scores among patients with intracranial stenosis, cerebral vascular reserve (CVR) <10% and cognitive dysfunction, found significantly improved cognitive function in patients after an STA-MCA bypass compared with those who were treated by other modalities.16)
However, we did not find any reports describing patients with acute and chronic cognitive impairment that improved after PTA. The SAMMPRIS trial found no significant differences in MoCA scores between groups treated medically and with stents.8) Another small study found that intracranial stents deployed in patients with reduced cerebral blood flow improved cerebral blood flow but not cognitive function.9)
We decided to treat our patient early because he had repeated neurological deficits and reduced cerebral blood flow determined after admission. Bypass surgery is also useful for such patients with hemodynamic compromise. However, our patient did not undergo SPECT with acetazolamide challenge, in reference to the SEE-JET trial because only 3 weeks had elapsed since his last ischemic attack,17) and an indication for bypass surgery was hardly judged. In addition, Mori A lesions suggested that PTA would result in a better outcome.18) After deep deliberation among neurologists, neurosurgeons and his family, PTA was finally decided. We guided a PTA balloon into a siphon, using an intermediate catheter and an over-the-wire-type balloon microcatheter, which essentially simplified crossing the wire into the lesion and increased the stability of the PTA.
Conclusion
We described a patient who underwent cerebral endovascular therapy to normalize cerebral blood flow during the acute phase of cognitive dysfunction and achieved improvement. Although medical treatment is practical for most patients with intracranial stenosis, some might have cognitive decline that is undetectable by simple screening. In patients with severe intracranial arterial stenosis, higher brain function should be screened very carefully, since cognitive decline might not be evident at the time of initial presentation. More evidence is needed to determine novel treatment strategies for targeted patients with cognitive decline that might be due to intracranial stenosis.
Disclosure Statement
None.
References
- 1). Dearborn JL, Zhang Y, Qiao Y, et al. : Intracranial atherosclerosis and dementia: The Atherosclerosis Risk in Communities (ARIC) Study. Neurology 2017; 88: 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Suri MFK, Zhou J, Qiao Y, et al. : Cognitive impairment and intracranial atherosclerotic stenosis in general population. Neurology 2018; 90: e1240–e1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Martinić-Popović I, Lovrencić-Huzjan A, Demarin V: Assessment of subtle cognitive impairment in stroke-free patients with carotid disease. Acta Clin Croat 2009; 48: 231–240. [PubMed] [Google Scholar]
- 4). Pucite E, Krievina I, Miglane E, et al. : Influence of severe carotid stenosis on cognition, depressive symptoms and quality of life. Clin Pract Epidemiol Ment Health 2017; 13: 168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Everts R, Wapp M, Burren Y, et al. : Cognitive and emotional effects of carotid stenosis. Swiss Med Wkly 2014; 144: w13970. [DOI] [PubMed] [Google Scholar]
- 6). Lal BK, Dux MC, Sikdar S, et al. : Asymptomatic carotid stenosis is associated with cognitive impairment. J Vasc Surg 2017; 66: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 7). Oudeman EA, Kappelle LJ, Van den Berg-Vos RM, et al. : Cognitive functioning in patients with carotid artery occlusion; a systematic review. J Neurol Sci 2018; 394: 132–137. [DOI] [PubMed] [Google Scholar]
- 8). Turan TN, Smock A, Cotsonis G, et al. : Is there benefit from stenting on cognitive function in intracranial atherosclerosis? Cerebrovasc Dis 2017; 43: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Kwon JY, Han YS, Kim JY, et al. : Intracranial artery stenting may not improve cognitive function: a preliminary study. J Stroke 2016; 18: 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Akiyama Y, Haruki Y, Kobayashi S: Cerebrovascular stenotic lesions and their severity and prognosis of disease type. Japanese Stroke Data Bank 2009 (in Japanese). Tokyo: Nakayama Shoten; 2009: 72–73. [Google Scholar]
- 11). Kasner SE, Chimowitz MI, Lynn NJ, et al. : Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–563. [DOI] [PubMed] [Google Scholar]
- 12). SSYLVIA Study Investigators : Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA): study results. Stroke 2004; 35: 1388–1392. [DOI] [PubMed] [Google Scholar]
- 13). Chimowitz MI, Lynn MJ, Derdeyn CP, et al. : SAMMPRIS trail investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Eng J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Zaidat OO, Fitzsimmons BF, Woodward BK, et al. : Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015; 313: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 15). Watanabe J, Ogata T, Higashi T, et al. : Cognitive change 1 year after CEA or CAS compared with medication. J Stroke Cerebrovasc Dis 2017; 26: 1297–1305. [DOI] [PubMed] [Google Scholar]
- 16). Ishikawa M, Kusaka G, Terao S, et al. : Improvement of neurovascular function and cognitive impairment after STA-MCA anastomosis. J Neurol Sci 2017; 373: 201–207. [DOI] [PubMed] [Google Scholar]
- 17). Ogasawara K, Ogawa A. JET study (Japanese EC-IC Bypass Trial). Nihon Rinsho 2006; 64:524–527. [PubMed] [Google Scholar]
- 18). Mori T, Fukuoka M, Kazita K, et al. : Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol 1998; 19: 1525–1533. [PMC free article] [PubMed] [Google Scholar]