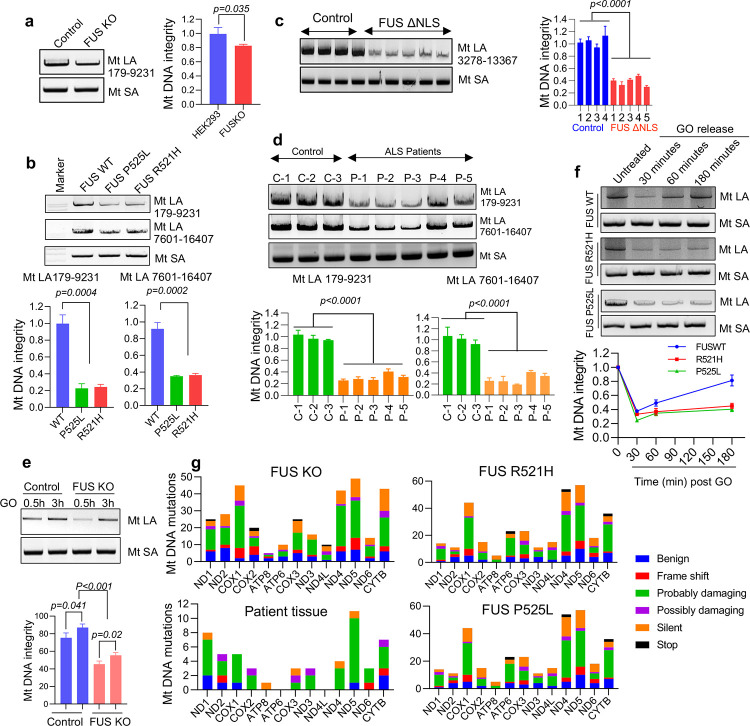
Figure 3: Accumulation of DNA damage and deficient DNA damage repair in ALS patient-derived FUS mutant cells.
a to f shows long amplification PCR (LA-PCR) to determine genomic integrity of mt DNA, >8000 bp fragment of mtDNA was amplified and separated in 1% agarose gel along with a control PCR of 200 bp (MtSA). Amplified PCR product is quantified by using pico green fluorescence a shows comparison between the control and FUS KO HEK293 cells, b shows comparison of genomic integrity between control and patient derived mutant fibroblasts using two amplicons. c represents the comparison of genomic integrity between WT mice and humanized FUS ΔNLS mice brain tissue at around 12-month age, d corresponds to human spinal cord tissue samples between control and ALS patient spinal cord with FUS pathology. e, and f differentiate the ability of FUS mutated cells and of FUS KO cells to repair oxidative DNA damage e shows patient derived fibroblasts whereas f represents HEK 293 cells. g corresponds to mitochondrial DNA sequencing in HEK 293 FUS KO cells, patient spinal cord tissues and patient derived fibroblasts performed using mitochondrial REPLI-g kit. The unique mutations in protein coding genes are represented based on the kind of mutation and the severity. The frame shift represents either insertions or deletions. Silent mutations showed coding for same amino acid, and the severity of mutation was determined either benign, probably damaging and possibly damaging based on polyphen2 analysis.