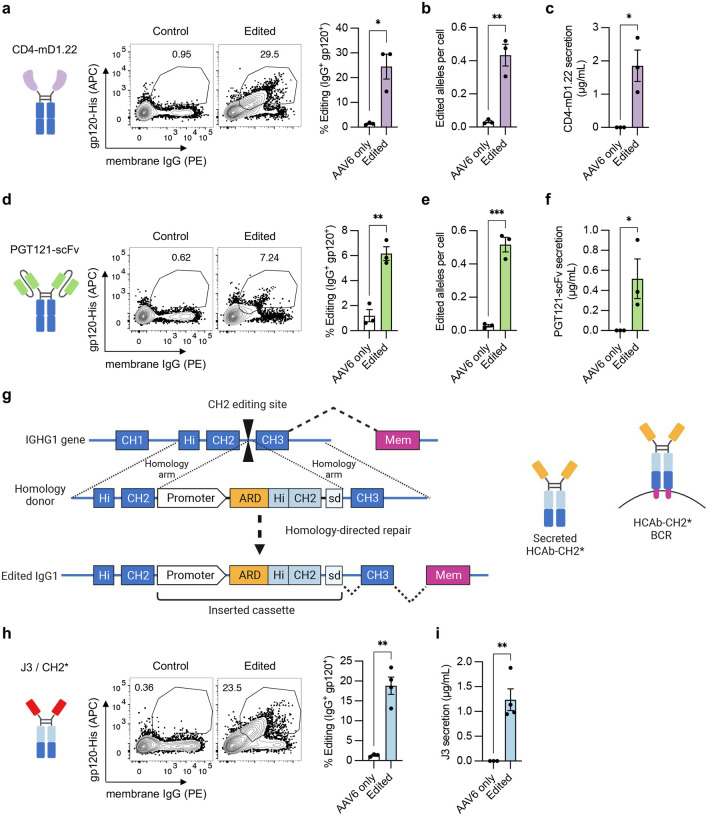
Figure 6. Editing with alternative HCAb structures and modified Fc domains.
(a-f) Primary human B cells from n = 3 independent experiments were activated with BAC in XF media, starting at day −3, and edited at day 0 with sg05 Cas9 RNPs and AAV6-CD4 donors (a-c, MOI = 5 × 105 vg/cell) or AAV6-PGT121-scFv donors (d-f, MOI = 5 × 105 vg/cell). (a,d) Editing rates were measured at day 8 by flow cytometry for surface J3-BCR. (b,e) Editing was quantified by in-out ddPCR at day 8 and normalized per cell against a control reaction. (c,f) HCAb secretion was measured by gp120-IgG ELISA at day 8. (g) Design of CH2 editing approach. Cas9 gRNA CH2-g1 targets the intron downstream of CH2. Homology donor cassette contains B cell specific promoter, antigen recognition domain (ARD), codon-wobbled IgG1 Hinge (Hi) and CH2 exons, and splice donor to link to endogenous CH3 and membrane exons after insertion. (h-i) Primary human B cells from n = 3–4 independent experiments were activated with BAC in XF media, starting at day −3, and edited at day 0 with CH2-g1 Cas9 RNPs and AAV6-J3-CH2* donor (MOI = 104 vg/cell). (h) Editing rates were measured at day 8 by flow cytometry for surface J3-BCR. (i) J3 HCAb secretion was measured by gp120-IgG ELISA at day 8. Error bars show mean ± SEM. Statistics were calculated by 2-tailed t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.