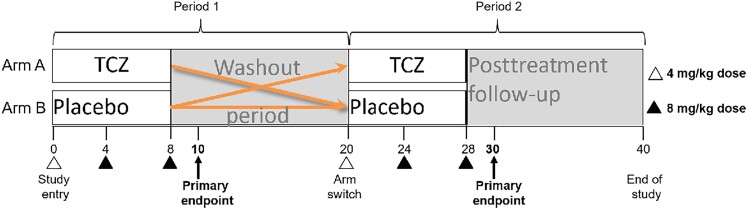
Figure 1.
Cross-over trial schema for interleukin 6 blockade with tocilizumab (TCZ) in people with human immunodeficiency virus (HIV). All participants had HIV, were aged 18–60 years, and had suppressed viremia on stable antiretroviral therapy. Study participants were randomized to receive TCZ (arm A) or placebo (arm B) intravenously during the first treatment period (10 weeks). The initial TCZ dose was 4 mg/kg; the second and third doses were 8 mg/kg and were given every 4 weeks. After a washout period, participants were switched to the opposite treatment and were followed through the end of study at 40 weeks. Three of 34 participants discontinued treatment, 1 due to low plasma antiviral drug levels (while receiving placebo, before receiving TCZ) and 2 due to dose limiting toxicities (1 grade 3 rash that occurred after the second dose of TCZ and 1 absolute neutrophil count <500 cells/µL after the first dose of TCZ).