Figure 2.
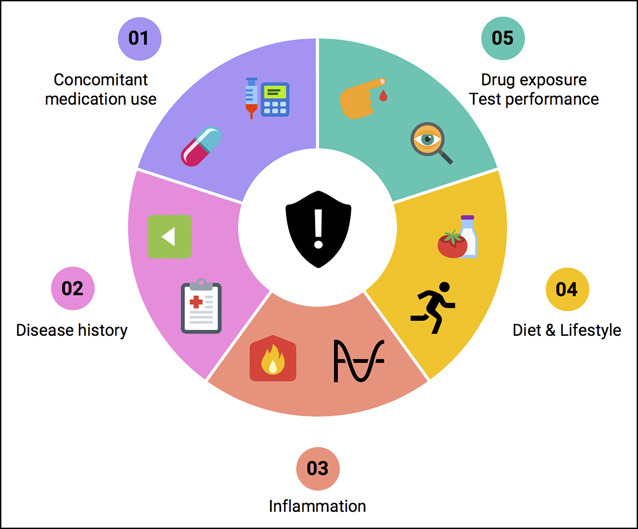
Key clinical confounders jeopardizing observations in multi-omics-driven biomarker studies. Important albeit non-exhaustive clinical factors warranting consideration in multi-omics studies include (1) concomitant medication use, which may induce molecular changes influencing the biological data under investigation; (2) disease history, including previous drug exposure but also disease duration and surgical history; (3) baseline inflammatory status, which may impact biological features and the outcome(s) under investigation; (4) diet, lifestyle, and environmental exposures, which may have an underrecognized influence on disease pathobiology; (5) drug exposure and test performance, i.e. is the drug under investigation biologically available (pharmacodynamic and pharmacokinetic properties) or in case of diagnostics, is the diagnostic method under investigation (e.g. endoscopy) appropriately performed and quantified to allow accurate investigation? Clinical disease heterogeneity of IBD poses a major challenge to multi-omics-driven biomarker discovery, necessitating stratification and the use of large, well-characterized patient and control cohorts.
