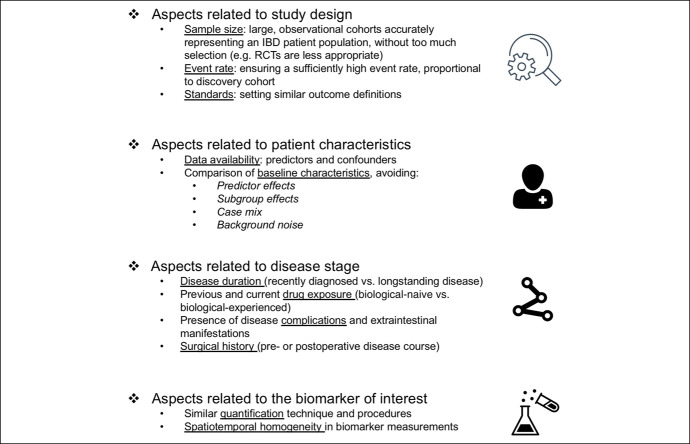
Figure 3.
Proposed criteria to consider when performing external validation of predictive signatures derived from multi-omics studies. Central criteria constitute aspects related to study design, including the sample size and sample characteristics (avoiding selection bias), but also the (proportionality of) event rate and definition of outcome parameters. Subsequently, patient and disease characteristics need to be carefully compared with those of the discovery cohort, while having the necessary predictors and confounding variables at hand in both cohorts. This should be performed to avoid predictor and subgroup effects, “case mix”, and background noise in study populations. Finally, it is critical to perform multi-omics data generation or quantification of the biomarker of interest in a similar manner, avoiding large differences in measurement circumstances or protocols.