Abstract
Purpose
Long interspersed nuclear element-1 (LINE-1 or L1) comprises 17% of the human genome. Retrotransposons may perturb gene integrity or alter gene expression by altering regulatory regions in the genome. The germline employs a number of mechanisms, including cytosine methylation, to repress retrotransposon transcription throughout most of life. Demethylation during germ cell and early embryo development de-represses retrotransposons. Intriguingly, de novo genetic variation appearing in sperm has been implicated in a number of disorders in offspring, including autism spectrum disorder, schizophrenia, and bipolar disorder. We hypothesize that human sperm exhibit de novo retrotransposition and employ a new sequencing method, single cell transposon insertion profiling by sequencing (scTIPseq) to map them in small amounts of human sperm.
Methods
Cross-sectional case–control study of sperm samples (n=10 men; ages 32–55 years old) from consenting men undergoing IVF at NYU Langone Fertility Center. scTIPseq identified novel LINE-1 insertions in individual sperm and TIPseqHunter, a custom bioinformatics pipeline, compared the architecture of sperm LINE-1 to known LINE-1 insertions from the European database of Human specific LINE-1 (L1Hs) retrotransposon insertions (euL1db).
Results
scTIPseq identified 17 novel insertions in sperm. New insertions were mainly intergenic or intronic. Only one sample did not exhibit new insertions. The location or number of novel insertions did not differ by paternal age.
Conclusion
This study for the first time reports novel LINE-1 insertions in human sperm, demonstrating the feasibility of scTIPseq, and identifies new contributors to genetic diversity in the human germ line.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02852-6.
Keywords: TIPseq, LINE-1, Human sperm, Aging
Introduction
Transposable elements (TEs) constitute a significant part of the human genome. TEs are subdivided into two main classes: retrotransposons (class I) that use the “copy and paste” process for their replication and expansion and DNA transposons (class II) that use the “cut and paste” mechanism [1]. Retrotransposons are classified into two categories: LTR (long terminal repeat), including endogenous retroviruses (ERVs), and non-LTR transposons, including LINEs (long interspersed elements), SINEs (short interspersed elements) and in humans the SINE-VNTR-Alu (SVA) [1]. Retrotransposons transcribe an intermediate RNA, which is then transcribed back into DNA and inserted into new locations within the genome, a process called retrotransposition [1]. In the mammalian genome, most retrotransposons have become incompetent for transposition, but some retrotransposons, especially LINEs, can become active [2]. Moreover, repressive epigenetic marks inhibit LINE-1 retrotransposition.
Cytosine demethylation removes most of the repressive epigenetic marks [3–5] during gamete formation and early embryo development. This epigenetic reprograming, geared towards more transcriptionally active chromatin, also promotes LINE-1 retrotransposition [6–8]. Retrotransposon activation may influence genomic stability by disrupting coding regions, or more commonly, regulatory regions in the host genome [8–13].
Male gametes develop more copy number variants (CNVs) compared to female gametes [14, 15] and paternally derived translocations arise more commonly in the offspring of older men [16, 17]. More generally, age alters chromatin structure and post transcriptional gene regulation pathways in many cell types [18–20]. With the age of parenthood increasing in most societies [21], understanding the effects of advancing paternal-age on the genomes’ offspring becomes an urgent priority. De novo genetic variation [22–24] [25, 26] contributes to a number of neuropsychiatric syndromes, including autism, schizophrenia, and bipolar affective disorder [27, 28]. Altered DNA methylation has been found in both somatic and germ cells [29] and may mediate some of the paternal age effects [30]. Similarly, LINE-1 becomes more transcriptionally active during aging and senescence [31, 32].
The precise location of de novo LINE-1 insertions is vital to understand their potential role as a source of structural variation and mutation. Because LINE-1 is a highly repetitive sequence found in high copy number throughout the human genome, identification of de novo LINE-1 insertions has been technically challenging. Transposon insertion profiling by next-generation SEQequencing (TIP-seq), and a machine-learning computational pipeline, TIPseqHunter, a next-generation sequencing-based method, were designed to overcome these challenges [33]. Recently this technique was adapted to profile LINE-1 insertions with limited amounts of DNA available for analysis, including individual cells [34]. The present study applied scTIPseq, and its associated bioinformatics pipeline, TIPseqHunter, to identify and map novel LINE-1 insertions in human sperm.
Material and methods
Study population and sample preparation
Cross-sectional case–control study including sperm samples (n=10; ages 32–55 years old) obtained from consenting ten men undergoing in vitro fertilization at NYU Langone Fertility Center, between February to July 2020. Sperm parameters were evaluated (Table 1), and samples washed for 10 min and re-suspended in global G1 medium. Sperm genomic DNA was extracted using DNeasy Blood and Tissue Kit (QIAGEN) following manufacturer’s recommended protocol, with an additional lysis step using X2 buffer (20 mM Tris-Cl, pH 8.0, 20 mM EDTA, 200 mM NaCl, 4% SDS); 80 mM dithiothreitol (DTT) and 12.5 μL/mL proteinase K [35]. DNA concentration and purity were measured using Qubit Fluorometer (ThermoFisher Scientific, cat. no. Q33226) (Table 1). Samples were stored at −80°C until TIPseq method.
Table 1.
Descriptive data of each semen parameters and DNA concentration pre and post multiple displacement amplification (MDA)
| Sample ID | Age (years) | Concentration (mL) | Progressive motility (%) | DNA concentration (ng/ul) pre MDA | DNA concentration (ng/ul) post MDA |
|---|---|---|---|---|---|
| 20163 | 55 | 48 | 51 | 20.4 | 86.0 |
| 13613 | 45 | 211 | 83 | 15.6 | 88.0 |
| 20196 | 32 | 140 | 57 | 20.0 | 78.4 |
| 20171 | 34 | 76 | 55 | 29.0 | 92.6 |
| 20120 | 32 | 78 | 79 | 31.6 | 79.0 |
| 20132 | 33 | 160 | 62 | 31.0 | 76.4 |
| 20145 | 49 | 53 | 44 | 25.8 | 83.6 |
| 20146 | 32 | 79 | 58 | 28.2 | 82.6 |
| 20173 | 55 | 90 | 44 | 33.4 | 90.2 |
| 20199 | 49 | 42 | 31 | 33.2 | 98.8 |
ID Identification; % percentage; concentration (×106/mL)
Whole genome amplification and quality control
Single-cell TIPseq was performed according to a recently published protocol [36], but adapted for use with low input DNA [34] as we were unable to get large amount of sperm DNA even using bulk sperm sample (Table 1). Briefly, samples were diluted to 10 ng in 2.5 ul and submitted to a preliminary step — multiple displacement amplification (MDA) using REPLI-g single cell kit (QIAGEN, cat no. 150343), to produce larger quantities of high quality DNA necessary for the TIPseq assay (Table 1). MDA provide a high level of coverage across the genome [37]. Placenta genomic DNA was used as positive control and double distilled water used as negative control. MDA products were purified following the protocol of the purification kit Agencourt AMPure XP Magnetic Beads (Beckman Coulter, cat. no. A63882 and DynaMag-TM Side Magnetic -ThermoFisher Scientific, cat.no. 12331D) and eluted in 50 μL of TE buffer. A qPCR was performed as a quality control (QC) to evaluate the efficiency of the MDA. The purified products were aliquoted into each well of a 96-well plate to perform the quantitative real-time PCR. Each reaction included 10 μL 2× SYBR Green mix (Bio-Rad), 2 μL each of 4 μM forward and reverse primers, 1 μL molecular grade water, and 5 μl diluted (1:300) MDA product, to yield a 20μL reaction. For this, twelve pairs of primers were designed for qPCR to amplify regions downstream of the 3’ end of fixed L1s insertions on different chromosomes (Supplementary table S1). Primers were synthesized by Integrated DNA Technologies (IDT). A Bio-Rad thermocycler (CFX96) was used to run program “CFX_3stepAMP” with anneal temperature set as 60°C. If all 12 regions are amplified, the sample is suitable to continue to the next step.
Vectorette PCR
An initial amount of 10 μg of the whole-genome amplified DNA was divided into aliquots that were independently digested with a panel of restriction enzymes: AseI (cat. no. R0526S), BspHI (cat. no. R0517S), BstYI (no. R0523S), HindIII (cat. no. R0104S), NcoI (cat. no. R0193S), and PstI (cat. no. R0140S) (NewEngland Biolabs). A pair of vectorette oligonucleotides (synthesized by IDT) corresponding to each restriction enzyme or T tail was annealed to form vectorette adaptors with a sticky end created (Supplementary table S2) [36]. Then the digested amplified genomic DNA were ligated with the vectorette adaptors using T4DNA ligase (NewEngland Biolabs, M0202S) at 4°C overnight. After ligation, PCR was performed with the L1 primer (5’- AGATATACC TAATGC TAG ATG ACACA -3’) and the vectorette primer (5’- CTC TCC CTT CTC GGATCT TAA -3’), using Hot-Start Taq DNA polymerase (Takara, cat no. RR006A) with a touchdown program (95°C 5 min; 5 cycles: 95°C 1 min, 72°C 1 min, 72°C 5 min, 5 cycles: 95°C 1 min, 68°C 1 min, 72°C 5 min, 5 cycles: 95°C 45 s, 64°C 1 min, 72°C 5 min, 15 cycles: 95°C 45 s, 60°C 1 min, 72°C 5 min, 72°C 15 min; 16°C hold).
DNA purification and quality control
PCR products were purified using the purification kit (Agencourt AMPure XP beads and DynaMagTM-96 Side Magnet) and were eluted in 21 μL of TE buffer. DNA concentration was measured using Qubit. An amount of 2 ug of purified DNA on 1.5% agarose — 1× TAE gel stained with SYBR Green was run at constant 100 V for 1 h or until separation of the ladder was clearly visible. Vectorette PCR amplicons should appear as a smear on the gel averaging around 1–3kb.
Next-generation sequencing
About 2 μg of the vectorette PCR products were sheared to fragments of around 300 bp. Sequencing libraries were prepared using the KAPA HTP DNA Library preparation Kit (Roche, cat no. KK8234). Libraries were sequenced on an Illumina HiSeq 4000 with paired-end 150 bp reads. Sequencing was performed in NYU Langone’s Department of Systems Genetics [34].
Data analysis using TIPseqHunter
The bioinformatics pipeline, TIPseqHunter [33], was performed by the Bioinformatics Core from the NYU Genomics Center, with oversight by Professor David Fenyo, the computational biologist who developed TIPseqHunter. TIPseqHunter is comprised of five steps: (i) sequence read preparation and quality control, (ii) sequence alignment, (iii) candidate LINE-1 insertion site identification, (iv) LINE-1 insertion site modeling, and (v) LINE-1 insertion site prediction. Because the efficacy of the PCR assay and the type of background associated with each TIPseq dataset can vary, the machine-learning algorithm is run on each sample. The reliability of single cell TIPseq results from sperm samples was calculated using kappa coefficient to compare the total number of LINE-1 insertions and insertions into developmentally expressed genes. These parameters were compared between sperm samples from young and older men, and also with reference parameters from European database of human specific LINE-1 (L1Hs) retrotransposon insertions in humans (euL1db).
Validation of unknown insertions
Validation of the unknown insertions was performed using the sperm samples after MDA, by 3’ junction PCR [34]. The 3’ junction PCR is designed to amplify the 3’ of LINE-1 insertion and the downstream flanking sequence using LINE-1 primer in the 3’ of LINE-1 and the other primer in unique flanking sequence. The primers were designed using an in silico PCR (https://primer3.ut.ee/). The 20ul PCR reaction was set up with 50ng of DNA, 2ul of 10× ExTaq buffer, 1.6ul of dNTP mix, 0.1ul of Ex Taq HS, 0.4ul of L1 primer (10uM), and 4ul of left or right primer (1uM), and amplified on the thermocycler with setting as 1 cycle of 94°C for 5 min; 30 cycles of 94°C for 30 s, 60°C for 30 s, 68°C for 30 s; 1 cycle of 68°C for 7 min. PCR product then were loaded on 0.5% agarose gel for electrophesis in 1X TAE buffer to identify amplifications.
Statistical analysis
The descriptive values of the samples were calculated through measurements of central position and dispersion. The median was used as central position measures and the interquartile range (25–75), and the minimum and maximum values of the samples included in study were used as dispersion measures.
Analyzes were implemented in the SAS version 9.4 program.
Results
Baseline parameters of the samples included in this study
Sample characteristics are shown in Table 2 as median (interquartile range — IQR). The median age of the 10 samples included in this study was 39.5 (32–55) years, the concentration of spermatozoa per ml was 78.5 million (42-211), and the percentage of progressive motility was 56 (31–83). The median age of the 5 samples < 35 years was 32 (32–34) years old, the concentration of spermatozoa per ml was 79 (76–160), and the percentage of progressive motility was 58 (55–79). The median age of the 5 samples ≥ 45 years was 49 (45–55) years old, the concentration of spermatozoa per ml was 53 (42–211), and the percentage of progressive motility was 44 (31–83).
Table 2.
Baseline parameters of the samples included in this study
| Samples | N | Age (years) | Concentration per ml | Progressive motility (%) |
|---|---|---|---|---|
| All samples | 10 | 39.5 (32–49) | 78.5 (53–140) | 56 (44–62) |
| ≤ 35 years | 5 | 32 (32–33) | 79 (78–140) | 58 (57–62) |
| ≥ 45 years | 5 | 49.0 (49–55) | 53 (48–90) | 44 (44–51) |
Data presented as median (interquartile range — IQR); N number, concentration (x106/mL); % percentage
LINE-1 insertions in sperm samples
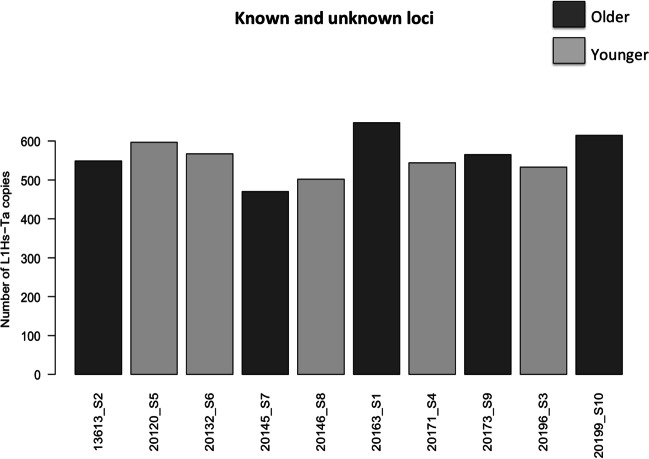
Total counts of LINE-1 insertions are shown in Fig. 1 and Table 3 as median (interquartile range — IQR). The median of all insertions observed in the 10 samples included in this study was [n: total number; median (minimum–maximum)]: n: 4831; 480.5 (415–565), the median of the known insertions n: 4606; 462 (400–538), the median of the unknown insertions n: 225; 24.5 (12–37) and, the median of the unique insertions n: 17; 1.00 (0–4). The median of all insertions found among the 5 samples age < 35 years included in this study was 476 (442–505), the median of the known insertions 457 (429–480), the median of the unknown insertions 24 (12–26), and the median of the unique insertions 1 (1–3). The median of all insertions found among the 5 samples age ≥ 45 years included in this study was 492 (415–565), the median of the known insertions 462 (400–538), the median of the unknown insertions 27 (15–37) and, the median of the unique insertions 1 (0–4).
Fig. 1.
Total count of LINE-1 insertions in sperm samples
Table 3.
LINE-1 insertions found on the samples included in this study
| Samples | N | Age (years) | All insertions | Known insertions | Unknown insertions | Unique insertions |
|---|---|---|---|---|---|---|
| All samples | 10 | 41.5 (32–55) | 480.5 (453–505) | 462 (30–480) | 24.5 (15–27) | 1.5 (1–3) |
| ≤ 35 years | 5 | 32 (32–33) | 476 (453–483) | 457 (430–463) | 24 (13–25) | 1 (1–3) |
| ≥ 45 years | 5 | 55 (49–55) | 492 (478–522) | 462 (462–485) | 27 (16–30) | 2 (1–4) |
Data presented as median (interquartile range — IQR); N number
Novel LINE-1 insertions
TIPseq identified 17 novel insertions in sperm, 8 from older (≥ 45 years) and 9 from younger men (< 35 years) (Fig. 1). New insertions were mainly intergenic or intronic, including TMEM163 (2/7), CTTNBP2NL (3/5), TMC2 (2/19), MACROD2 (2/6), RAB3C (3/4), LINC02664 (1/1), AC007402 (2/10), AC107023 (3/3), AC079052 (2/3), and AC017091 (4/4) (Table 4). One novel insertion (< 35 years old) hits a known regulatory element. Only one sample (≥ 45 years old) did not exhibit any new insertion (Fig. 1, Table 4). The number of novel insertions did not differ by paternal age.
Table 4.
Descriptive data of the new and unique insertions found in sperm samples
| Sample ID | Age | Chromosome | Gene | Repeat | Regulatory element |
|---|---|---|---|---|---|
| 13613 | 45 | chr20 | |||
| 20120 | 32 | chr2 | AC007402 intron (2/10) | L1MB5 | |
| 20120 | 32 | chr2 | TMEM163 intron (2/7) | AluSp | |
| 20120 | 32 | chr13 | |||
| 20132 | 33 | chr1 | CTTNBP2NL intron (3 of 5) | AluJb | |
| 20132 | 33 | chr2 | |||
| 20132 | 33 | chr12 | AC107023 intron (3/3) | ||
| 20145 | 49 | chr20 | TMC2 Intron (2/19) | ||
| 20145 | 49 | chr20 | MacroD2 intron (2/6) | L1PB1 | CEBPB TF binding site |
| 20146 | 32 | chr4 | |||
| 20171 | 34 | chr5 | RAB3C intron (3/4) | L1PA8A | |
| 20173 | 55 | chr6 | L2 | ||
| 20196 | 32 | chr11 | Enhancer | ||
| 20199 | 49 | chr1 | Intergenic | L1PA13 | |
| 20199 | 49 | chr4 | LINC02664 intron (1/1) | L1ME3 | |
| 20199 | 49 | chr10 | AC079052 intron (2/3) | ||
| 20199 | 49 | chr18 | AC017091 intron (4/4) | ||
| 20163 | 55 | No new insertion |
Validation of unique insertions using PCR
We performed 3’ junction PCR validations on 5 of these 17 unknown insertions (primers sequences are shown in table S3, Fig. 2, 3, 4). Specific PCR amplicons aligned to the appropriate location in the genome. This is consistent with the findings of McKerrow et al. (2019) that TIPseq and TIPseqHunter analysis provide infrequent false-positive calls after filtering, showing near-complete profiles of LINE-1 insertion sites.
Fig. 2.
Novel and unique LINE-1 in sperm samples
Fig. 3.
Comparison of LINE-1 insertions and men’s age
Fig. 4.
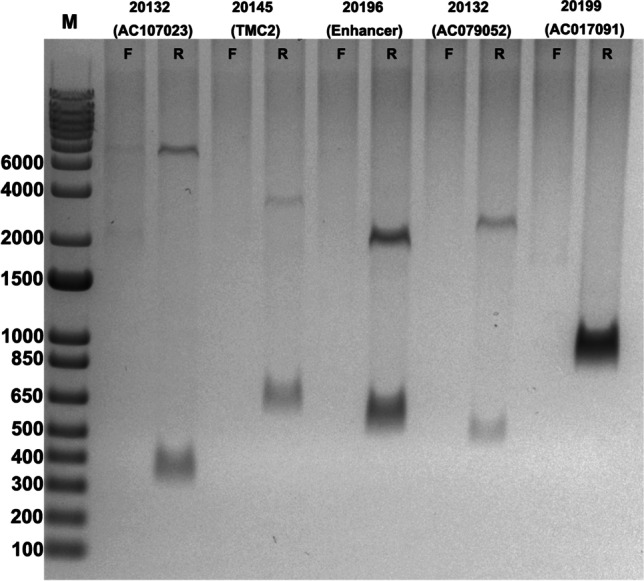
Validation of unique LINE-1 insertions using PCR
Discussion
Transposable elements shaped the human genome in the past and presumably continue to do so. The retrotransposon activity of LINE-1 is repressed during most stages of life, but epigenetic changes during gametogenesis and early embryo development can unleash activity to create novel insertions that provide structural variation to our genome [36]. The highly repetitive nature of these sequences make them especially challenging to locate within the genome [33], but here we apply a sensitive strategy to amplify and sequence LINE-1 retrotransposon insertions in human sperm using single cell transposon insertion profiling by next-generation sequencing (scTIPseq). Our study is the first to report novel LINE-1 insertions in human sperm, demonstrating that TIPseq is a feasible technique to identify and map LINE-1 insertions in human gametes.
For the first time, we report total counts of novel LINE-1 insertions in sperm samples. That specific PCR amplicons aligned to the proper genomic location of all five novels insertions validates our findings.
Our experiments did not identify increased novel LINE-1s in sperm from older men. The limited sample size and age ranges likely would be inadequate to identify age effects on novel LINE-1 insertions. Moreover, not all sperm within a sample may exhibit novel insertions. A small fraction of sperm within sample-inheriting novel insertions would not have been detected by the method we employed. Additional studies using larger samples sizes are needed to map insertions in a wider range of men.
In human and mouse sperm, EGFP expression indicated that retroelements can be internalized in sperm cells, independently of the sperm origin and penetrate the tightly packed chromatin [38–40]. This permeability is a distinctive feature of both, epididymal and ejaculated spermatozoa (after removing the seminal fluid), from all animal species including humans [41, 42]. Mouse spermatozoa not only can integrate exogenous retroelements, but they also transfer them into the oocyte during fertilization, affecting preimplantation embryo development [40]. To proper compare if the insertions occur during spermatogenesis or in mature epididymal spermatozoa, it would be necessary to compare sperm samples extracted from the testis and epididymis, which would be extremely challenging.
The occurrence of the integration suggests that the sperm chromosomal DNA is not uniformly and tightly packed with protamine, implying the existence of genomic sites where the chromosomal DNA is accessible to foreign molecules [43]. The integration of endogenous retrotransposons and of foreign DNA molecules is proposed to occur in the nucleohistone chromatin spacer within two adjacent protamine domains [43]. The nucleosomal sperm chromatin fraction interacts with the nuclear scaffold and is supplied with the enzymatic machinery required to support DNA integrations [43] that could interfere with the genome, possibly influence the sequence of certain loci, and give rise to new retrotransposition events [38–40].
According to NCBI database, some of the genes where these new and unique insertions were found have uncharacterized functions [44]. The transmembrane protein 163 gene (TMEM163) has been predicted to enable zinc ion–binding activity, to be involved in zinc ion import into synaptic vesicle, to be located in early endosome membrane, to be active in intracellular vesicle and plasma membrane, and to be integral component of synaptic vesicle membrane [44]. This gene also is associated with hypomyelination leukodystrophy genetic disorder [45]. The CTTNBP2 N-terminal like gene (CTTNBP2NL) located in actin cytoskeleton enables protein phosphatase 2A binding activity. Acts upstream of or within negative regulation of transmembrane transport [44]. Overexpression of CTTNBP2NL inhibited embryo implantation in mice whereas knockdown of CTTNBP2NL decreased phosphatase activity [46]. Regard the transmembrane channel like 2 gene (TMC2), mutations on this gene may underlie hereditary disorders of balance and hearing [44]. The protein encoded by mono-ADP ribosylhydrolase 2 gene (MACROD2) is a deacetylase involved in removing ADP-ribose from mono-ADP-ribosylated proteins. The encoded protein has been shown to translocate from the nucleus to the cytoplasm upon DNA damage [44]. Deletion in MACROD2 is suggested to affect correct embryonic development in a family with complex phenotypes such as microcephaly, intellectual disability, polydactyly, renal, and pancreatic malformations [47]. RAB3C, a member of the RAS oncogene family, encodes a small GTPase. Other similar small GTPases are known to be involved in vesicle trafficking, and the encoded protein was shown to play a role in recycling phagocytosed MHC class 1 complexes to the cell surface [44]. RAB3C gene was also suggested as a potential marker for post-thaw boars sperm quality for cryopreservation [48].
These associations are intriguing, but much remains to be understood regarding the novel LINE-1 insertions found by scTIPseq. We believe that new powerful analytical methods, such as scTIPseq, will help clarify the role of retrotransposition events in human gametes, and possibly to further elucidate the role of retrotransposons in sperm function and male infertility.
Supplementary information
(DOCX 18 kb)
Data accessibility
All the sequencing data are stored at the BigPurple cluster. TIPseqHunter2 software is available at https://github.com/FenyoLab/TIPseqHunter.
Funding
Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code: 001, Brazil; process no. 88887.371487/2019-00 and process n. 88887.597054/2021-00 to TSB), Brazilian National Council for Scientific and Technological Development (CNPq, Brazil; Grant number 204747/2018-0 to FBK) and the Stanley H. Kaplan Fund of the NYU Grossman School of Medicine (to DLK).
Data availability
The data underlying this study will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval
The New York University Langone Medical Center Institutional Review Board (IRB) approved this study under the IRB number S#16-00154.
Consent for publication
The author agree to its submission to the Journal of Assisted Reproduction and Genetics and, if accepted, to its publication in this journal. We warrant that this article is original, does not infringe on any copyright or other proprietary right of any third party, is not under consideration by another journal and has not been previously published. Ethical approval has been sought and obtained as necessary and any conflicts of interest stated.
Consent to participate
Each participant provided written informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mita P, Boeke JD. How retrotransposons shape genome regulation. Curr Opin Genet Dev. 2016;37:90–100. doi: 10.1016/j.gde.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spadafora C. A LINE-1-encoded reverse transcriptase-dependent regulatory mechanism is active in embryogenesis and tumorigenesis. Ann N Y Acad Sci. 2015;1341:164–171. doi: 10.1111/nyas.12637. [DOI] [PubMed] [Google Scholar]
- 3.Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol. 2002;241:172–182. doi: 10.1006/dbio.2001.0501. [DOI] [PubMed] [Google Scholar]
- 4.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 5.Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110330. doi: 10.1098/rstb.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazazian HH, Jr, Moran JV. Mobile DNA in health and disease. N Engl J Med. 2017;377:361–370. doi: 10.1056/NEJMra1510092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solyom S, Kazazian J. Mobile elements in the human genome: implications for disease. Genome Med. 2012;4(2):12. 10.1186/gm311. [DOI] [PMC free article] [PubMed]
- 8.Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, et al. L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev. 2009;23:1303–1312. doi: 10.1101/gad.1803909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns KH, Boeke JD. Human transposon tectonics. Cell. 2012;149:740–752. doi: 10.1016/j.cell.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrell DT. Epigenetics of the male gamete. Fertil Steril. 2012;97:267–274. doi: 10.1016/j.fertnstert.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Kazazian HH, Jr, Genetics. L1 retrotransposons shape the mammalian genome. Science. 2000;289:1152–1153. doi: 10.1126/science.289.5482.1152. [DOI] [PubMed] [Google Scholar]
- 12.Kazazian HH. Retrotransposon insertions in germ cells and somatic cells. Dev Biol (Basel) 2001;106:307–313. [PubMed] [Google Scholar]
- 13.Kazazian HH., Jr Mobile elements: drivers of genome evolution. Science. 2004;303:1626–1632. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- 14.Lowe X, Collins B, Allen J, Titenko-Holland N, Breneman J, van Beek M, et al. Aneuploidies and micronuclei in the germ cells of male mice of advanced age. Mutat Res. 1995;338:59–76. doi: 10.1016/0921-8734(95)00012-u. [DOI] [PubMed] [Google Scholar]
- 15.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 16.Thomas NS, Morris JK, Baptista J, Ng BL, Crolla JA, Jacobs PA. De novo apparently balanced translocations in man are predominantly paternal in origin and associated with a significant increase in paternal age. J Med Genet. 2010;47:112–115. doi: 10.1136/jmg.2009.069716. [DOI] [PubMed] [Google Scholar]
- 17.Thomas NS, Durkie M, Van Zyl B, Sanford R, Potts G, Youings S, et al. Parental and chromosomal origin of unbalanced de novo structural chromosome abnormalities in man. Hum Genet. 2006;119:444–450. doi: 10.1007/s00439-006-0157-6. [DOI] [PubMed] [Google Scholar]
- 18.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood JG, Helfand SL. Chromatin structure and transposable elements in organismal aging. Front Genet. 2013;4:274. doi: 10.3389/fgene.2013.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell PH. What might retrotransposons teach us about aging? Curr Genet. 2016;62:277–282. doi: 10.1007/s00294-015-0538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bray I, Gunnell D, Davey Smith G. Advanced paternal age: how old is too old? J Epidemiol Community Health. 2006;60:851–853. doi: 10.1136/jech.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra D, McCarthy S, Michaelson JJ, Vacic V, Burdick KE, Yoon S, et al. High frequencies of de novo CNVs in bipolar disorder and schizophrenia. Neuron. 2011;72:951–963. doi: 10.1016/j.neuron.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;488:471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flatscher-Bader T, Foldi CJ, Chong S, Whitelaw E, Moser RJ, Burne TH, et al. Increased de novo copy number variants in the offspring of older males. Transl Psychiatry. 2011;1:e34. doi: 10.1038/tp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;2:245–261. doi: 10.1016/s1568-1637(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 30.Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull. 2001;27:379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Cecco M, Criscione SW, Peckham EJ, Hillenmeyer S, Hamm EA, Manivannan J, et al. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell. 2013;12:247–256. doi: 10.1111/acel.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Cecco M, Criscione SW, Peterson AL, Neretti N, Sedivy JM, Kreiling JA. Transposable elements become active and mobile in the genomes of aging mammalian somatic tissues. Aging (Albany NY) 2013;5:867–883. doi: 10.18632/aging.100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Z, Steranka JP, Ma S, Grivainis M, Rodic N, Huang CR, et al. Human transposon insertion profiling: analysis, visualization and identification of somatic LINE-1 insertions in ovarian cancer. 2017;114:E733–e40. 10.1073/pnas.1619797114. [DOI] [PMC free article] [PubMed]
- 34.McKerrow W, Tang Z, Steranka JP, Payer LM, Boeke JD, Keefe D, et al. Human transposon insertion profiling by sequencing (TIPseq) to map LINE-1 insertions in single cells. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190335. doi: 10.1098/rstb.2019.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antunes DM, Kalmbach KH, Wang F, Dracxler RC, Seth-Smith ML, Kramer Y, et al. A single-cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet. 2015;32:1685–1690. doi: 10.1007/s10815-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steranka JP, Tang Z, Grivainis M, Huang CRL, Payer LM, Rego FOR, et al. Transposon insertion profiling by sequencing (TIPseq) for mapping LINE-1 insertions in the human genome. Mob DNA. 2019;10:8. doi: 10.1186/s13100-019-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coskun S, Alsmadi O. Whole genome amplification from a single cell: a new era for preimplantation genetic diagnosis. Prenat Diagn. 2007;27:297–302. doi: 10.1002/pd.1667. [DOI] [PubMed] [Google Scholar]
- 38.Mastora E, Christodoulaki A, Papageorgiou K, Zikopoulos A, Georgiou I. Expression of Retroelements in Mammalian Gametes and Embryos. In Vivo. 2021;35(4):1921–1927. doi: 10.21873/invivo.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazaros L, Kitsou C, Kostoulas C, Bellou S, Hatzi E, Ladias P, Stefos T, Markoula S, Galani V, Vartholomatos G, Tzavaras T, Georgiou I. Retrotransposon expression and incorporation of cloned human and mouse retroelements in human spermatozoa. Fertil Steril. 2017;107(3):821–830. doi: 10.1016/j.fertnstert.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 40.Kitsou C, Lazaros L, Bellou S, Vartholomatos G, Sakaloglou P, Hatzi E, Markoula S, Zikopoulos K, Tzavaras T, Georgiou I. Exogenous retroelement integration in sperm and embryos affects preimplantation development. Reproduction. 2016;152(3):185–193. doi: 10.1530/REP-15-0174. [DOI] [PubMed] [Google Scholar]
- 41.Smith K, Spadafora C. Sperm-mediated gene transfer: applications and implications. Bioessays. 2005;27(5):551–562. doi: 10.1002/bies.2021. [DOI] [PubMed] [Google Scholar]
- 42.Spadafora C. Sperm cells and foreign DNA: a controversial relation. Bioessays. 1998;20(11):955–964. doi: 10.1002/(SICI)1521-1878(199811)20:11<955::AID-BIES11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Pittoggi C, Zaccagnini G, Giordano R, Magnano AR, Baccetti B, Lorenzini R, Spadafora C. Nucleosomal domains of mouse spermatozoa chromatin as potential sites for retroposition and foreign DNA integration. Mol Reprod Dev. 2000;56(2 Suppl):248–251. doi: 10.1002/(SICI)1098-2795(200006)56:2+<248::AID-MRD7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Available from: http://www.ncbi.nlm.nih.gov/guide/genes-expression/. Accessed 23 Jan 2023.
- 45.Yan H, Yang S, Hou Y, Ali S, Escobar A, Gao K, et al. Functional study of TMEM163 gene variants associated with hypomyelination leukodystrophy. Cells. 2022:11. 10.3390/cells11081285. [DOI] [PMC free article] [PubMed]
- 46.Sugimoto M, Sasaki S, Gotoh Y, Nakamura Y, Aoyagi Y, Kawahara T, et al. Genetic variants related to gap junctions and hormone secretion influence conception rates in cows. Proc Natl Acad Sci U S A. 2013;110:19495–19500. doi: 10.1073/pnas.1309307110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombardo B, Esposito D, Iossa S, Vitale A, Verdesca F, Perrotta C, et al. Intragenic deletion in MACROD2: a family with complex phenotypes including microcephaly, intellectual disability, polydactyly, renal and pancreatic malformations. Cytogenet Genome Res. 2019;158:25–31. doi: 10.1159/000499886. [DOI] [PubMed] [Google Scholar]
- 48.Mańkowska A, Brym P, Paukszto Ł, Jastrzębski JP, Fraser L. Gene polymorphisms in boar spermatozoa and their associations with post-thaw semen quality. Int J Mol Sci. 2020:21. 10.3390/ijms21051902. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)
Data Availability Statement
The data underlying this study will be shared on reasonable request to the corresponding author.