Table 5.
The obtained optimisation parameters for the Suzuki C–C coupling reactiona.
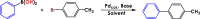 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst (mmol) | Base | Solvent | Temperature (°C) | Time (h) | Conversion (%)b | Yield (%)b | Selectivity (%) | TONc |
| 1 | 0.005 | KOH | DMF | 80 | 12 | >13 | >1 | >11 | >27 |
| 2 | 0.005 | K2CO3 | DMF | 80 | 12 | >47 | >28 | >60 | >94 |
| 3 | 0.005 | K3PO4 | DMF | 80 | 12 | >14 | >1 | >11 | >28 |
| 4 | 0.005 | Na2CO3 | DMF | 80 | 12 | >41 | >20 | >48 | >83 |
| 5 | 0.005 | Cs2CO3 | DMF | 80 | 12 | >13 | >0 | >3 | >27 |
| 6 | 0.005 | Et3N | DMF | 80 | 12 | >43 | >2 | >5 | >87 |
| 7 | 0.005 | K2CO3 | DMF | 90 | 12 | >46 | 28 | >60 | >92 |
| 8 | 0.005 | K2CO3 | DMF | 100 | 12 | >50 | >35 | >65 | >100 |
| 9 | 0.005 | K2CO3 | DMF | 110 | 12 | 51 | 36 | 70 | 102 |
| 10 | 0.005 | K2CO3 | DMF | 120 | 12 | 52 | 36 | 69 | 104 |
| 11 | 0.005 | K2CO3 | H2O | 110 | 12 | 82 | >76 | >93 | 164 |
| 12 | 0.005 | K2CO3 | EtOH | 110 | 12 | 100 | >98 | >98 | 200 |
| 13 | 0.005 | K2CO3 | 1,4-Dioxane | 110 | 12 | 91 | >85 | 94 | 182 |
| 14 | 0.005 | K2CO3 | Toluene | 110 | 12 | >98 | >92 | >93 | >197 |
| 15 | 0.005 | K2CO3 | CH3CN | 110 | 12 | 100 | 94 | 94 | 200 |
| 16 | 0.005 | K2CO3 | Et4NBr-DMF | 110 | 12 | >58 | >49 | 84 | >197 |
| 17 | 0.005 | K2CO3 | EtOH– H2O | 110 | 12 | 100 | >94 | >94 | 200 |
| 18 | 0.005 | K2CO3 | 1,4-Dioxane-H2O | 110 | 12 | >97 | >94 | >97 | >194 |
| 19 | 0.005 | K2CO3 | EtOH | 110 | 2 | 96 | 95 | >98 | 192 |
| 20 | 0.005 | K2CO3 | EtOH–Et4NBr | 110 | 2 | 98 | 97d | >98 | 196 |
| 21 | 0.005 | K2CO3 | EtOH | 110 | 4 | 98 | 97 | >98 | 196 |
| 22 | 0.005 | K2CO3 | EtOH–Et4NBr | 110 | 4 | 98 | 97d | >98 | 196 |
| 23 | 0.001 | K2CO3 | EtOH | 110 | 0.5 | 86 | 86 | 100 | 860 |
| 24 | 0.001 | K2CO3 | EtOH | 110 | 5 | >97 | >97 | 100 | >970 |
| 25 | 0.0001 | K2CO3 | EtOH | 110 | 12 | 86 | 86 | 100 | 8600 |
| 26 | 0.0002 | K2CO3 | EtOH | 110 | 12 | 89 | 89 | 100 | 4450 |
Reaction circumstances: 4-Bromotoluene (1.0 mmol), phenylboronic acid (1.2 mmol), base (1.2 mmol), solvent (2 mL).
Yield and conversion rates were determined by GC analysis based on aryl halides in the presence of dodecane used as internal standard.
Mole product/mole catalyst.
Et4NBr (0.02 mmol).
