Abstract
C3H (H-2k) mice are susceptible to a vaginal challenge with human strains of Chlamydia trachomatis and thus are a useful strain for testing potential Chlamydia vaccine candidates. However, C3H mice are fairly poor responders in terms of the level of antibody resulting from immunization with potential protective peptides representing variable domains (VDs) of the major outer membrane protein (MOMP). C57BL/6 (H-2b) mice, on the other hand, are moderately resistant to a vaginal challenge but are good responders to the chlamydial MOMP VDs. Peptides representing universal T-cell helper epitopes were employed to determine whether the antibody response to a peptide representing VD4 of the MOMP, which has been shown to contain neutralizing epitopes, could be enhanced in C3H and C57 mice. Universal T-cell helper peptides from tetanus toxin, the pre-S2 region of hepatitis B virus, and the mouse heat shock protein 60, as well as the corresponding segment of the Chlamydia heat shock protein 60 (hspct), were coadministered with the VD4 peptide. Peptides were coencapsulated in liposomes containing the adjuvant monophosphoryl lipid A and administered by using a combination of mucosal and intramuscular injection. The only T-cell helper peptide that improved the immune response as judged by antibody level, in vitro neutralization assays, and T-cell proliferation was hspct. The response in the C57BL/6 strain was not significantly enhanced with hspct over levels achieved with VD4 alone; however, in C3H mice the levels of serum antibody to C. trachomatis increased to that seen in C57 mice. However, the molecular specificity and immunoglobulin subclass distribution differed from those of the C57 response, and the neutralizing titers and T-cell proliferation responses were lower. In both strains of mice, titers of vaginal antibody to C. trachomatis were low. In summary, of the T-helper peptides used, only hspct significantly enhanced the immune response of C3H mice to the VD4 peptide, but it had only a modest effect on the immune response of C57 mice.
Chlamydia trachomatis, being a leading cause of sexually transmitted diseases, has been the focus of efforts to develop a protective vaccine (4, 31, 37). Central to this effort has been the identification of host factors that may protect against infections caused by this pathogen as well as the definition of chlamydial components that confer pathogenic potential to this organism. To date the major outer membrane protein (MOMP) has been the most widely investigated vaccine candidate of the chlamydial proteins (17). Within the MOMP there are four variable domains (VDs), which differ among the serovars and are regions in which there are neutralizable epitopes (3, 17, 24, 30, 33, 40). In vitro experiments using monoclonal antibodies directed at the VDs have shown that attenuation of infection is narrow in terms of the number of serovars that are neutralized when any one epitope is targeted (24, 30, 40). Since neutralization of all serovars is not achieved by using antibodies directed at any one epitope, a subunit vaccine that incorporates an array of protective epitopes has been proposed (4, 31, 37). For the few attempts to utilize recombinant MOMP or peptides representing VD neutralizing epitopes to immunize mice, only modest attenuation of the infection has been reported (36, 38). There may be several reasons for this, including the requirement for T-cell help to boost and direct the immune response to critical regions within these peptides, the failure to elicit an adequate mucosal immune response, the requirement for a conformational epitope not provided by short synthetic peptides, and inherent differences in inbred mouse strains used in the vaccine trials.
It has been shown that mouse strains of different H-2 haplotypes vary dramatically in their responses to a genital challenge with C. trachomatis (8, 9). As an example, C57BL/6 (H-2b) mice are fairly resistant to a vaginal challenge with a human serovar, with vaginal cultures, depending on the challenge dose, being positive for only 1 to 2 weeks following inoculation. In contrast, C3H (H-2k) mice are infected with lower numbers of organisms, and C. trachomatis can be cultured from the vagina for up to 4 to 6 weeks following challenge (9, 26). Therefore, because of the longer duration of infection, C3H mice are an attractive strain in which to test peptide vaccine candidates. However, it has been shown that a peptide representing VD4 of the MOMP was immunogenic in C57BL/6 (H-2b) mice but was significantly less effective in eliciting a humoral response in C3H/HeJ and B10.BR/SgSnJ mice, both of which are of the H-2k haplotype (29, 34).
The purpose of this study was to determine whether universal T-cell helper peptides could enhance the immune response to a VD4 peptide in the otherwise nonresponsive C3H mouse or modify the response in C57 mice. Since the long-term goal is to protect against a genital mucosal challenge, the immunization strategy used was to coadminister, through a combination of systemic and mucosal routes, T-cell helper peptides and a VD4 peptide coentrapped in liposomes. Eliciting an immune response in the permissive but low- or nonresponsive C3H strain is essential for the future development and testing of subunit vaccines in this animal model.
MATERIALS AND METHODS
Organisms.
C. trachomatis, serovar E (BOUR), was obtained from the American Type Culture Collection (Rockville, Md.). The strain was grown in HeLa 229 cells (American Type Culture Collection), and elementary bodies (EBs) were purified as previously described (28).
Peptides.
Synthetic peptides, representing the VD4 and T-cell helper epitopes, are shown in Table 1. The peptides were made by 9-fluorenylmethoxycarbonyl synthesis at the University of California Microchemical Core Laboratory and were purified by high-pressure liquid chromatography.
TABLE 1.
Peptides used for immunization
| Peptide | Protein (amino acids) | Sequence (NH2→COOH) | Reference |
|---|---|---|---|
| VD4 | MOMP, serovar E (309–338) | C-SATAIFDTTTLNPTIAGAGDVKASAEGQLG | 25a |
| hspm | Mouse hsp60 (458–474) | NEDQKIGIEIIKRALKIa | 16 |
| hspct | Chlamydia hsp60 (435–451) | NEDEQIGARIVLKALSAa | 7 |
| TT | Tetanus toxin (830–844) | QYIKANSKFIGITEL | 21 |
| Pre-S2 | Hepatitis B surface antigen (120–132) | MQWNSTTFHQTLQ | 19 |
Underlined amino acids are homologous in the two hsps.
Liposomes.
Peptide entrapment in liposomes was carried out by the dehydration-rehydration procedure (15). Liposomes were composed of dimyristoyl phosphatidylcholine (Avanti Polar Lipids Inc., Alabaster, Ala.), dimyristoyl phosphatidylglycerol (Avanti), and cholesterol (Avanti) in a molar ratio of 1.8:0.2:1.5 and were supplemented by direct incorporation of monophosphoryl lipid A (MPLA) (Ribi, Hamilton, Mont.) (2). The dose of MPLA was 5 μg per route of immunization. Briefly, the liposomes were prepared by mixing 3.1 ml of dimyristoyl phosphatidylcholine (20 mg/ml), 0.3 ml of dimyristoyl phosphatidylglycerol (25 mg/ml), 2.9 ml of cholesterol (10 mg/ml), and 0.5 ml of MPLA (1 mg/ml). All compounds were dissolved in chloroform, except MPLA, which was solubilized in chloroform-methanol (4:1, vol/vol). The mixture was placed in a round-bottom flask, and chloroform-methanol was removed by using a rotating evaporator (R114; Buchi, Flawil, Switzerland). The dried lipid film was suspended in 5.5 ml of phosphate-buffered saline (PBS), sonicated (Braun-Sonic 2000; B. Braun Instruments, Burlingame, Calif.) for 40 min (15-s pulse per min) on ice, and clarified by centrifugation 5,000 × g for 10 min. The liposomes were mixed with the corresponding peptide(s) dissolved in PBS at a concentration of 4 mg/ml. Different peptide combinations mixed with liposomes were lyophilized separately, the resulting dried film was reconstituted with water to the volume prior to lyophilization, and this preparation was used to immunize mice.
To assay the efficiency of incorporation of peptides into liposomes, the above-described preparation was centrifuged for 1 h at 35,000 rpm with an SW60 rotor and an L2 ultracentrifuge (Beckman, Fullerton, Calif.). Concentrations of peptides in the supernatant as well as in a pellet lysed with Triton X-100 were measured by using a fluoraldehyde protein/peptide assay kit (Pierce, Rockford, Ill.). Reaction results were read at an excitation wavelength of 340 nm and an emission wavelength of 455 nm by using an LS-5B luminescence spectrometer (Perkin-Elmer, Foster City, Calif.). The efficiency of incorporation of antigen into liposomes ranged from 15 to 20%.
Immunization of mice.
Female 6- to 7-week-old C57BL/6 (H-2b) and C3H/HeBkI (H-2k) mice were purchased from B&K Universal Inc. (Fremont, Calif.). Groups of seven mice of each strain were immunized with the VD4 peptide alone or together with a T-cell peptide encapsulated in liposomes. Mice were immunized intramuscularly by administering 25 μg of the VD4 peptide alone or as a mixture with 25 μg of one of the T-cell peptides to both hind legs. Mice were boosted by both the presacral and intranasal routes 3 and 5 weeks after priming with the same dose as described above.
Immunoassays.
The indirect inclusion immunofluorescence assay (IFA) and enzyme-linked immunosorbent assay (ELISA) were performed as previously described (25, 27, 29). Wells of microtiter plates (Corning Glassworks, Corning, N.Y.) used for ELISA were coated with 1 μg of synthetic peptide or Renografin-purified EBs (5). Twofold serial dilutions of mouse sera were tested, and goat anti-mouse immunoglobulin labeled with horseradish peroxidase (Cappel, Organon Teknika Corp., Durham, N.C.) was used as a secondary antibody. Determination of class- and subclass-specific antibodies was performed by ELISA as previously described (25). Overlapping peptides representing the VD4 region of C. trachomatis serovar E were synthesized by the method of Geysen et al. (10) by using an epitope mapping kit (Cambridge Research Biochemicals, Cambridge, England). Pepscans were performed with pins containing the overlapping hexapeptides as previously described (24).
Competitive inhibition assay.
Inhibition studies were performed to determine what peptide(s) could block the recognition of EBs by IFA with antibodies obtained from mice immunized with coentrapped VD4 and hspct (Chlamydia heat shock protein 60 [hsp60]) peptides. Antisera diluted 1:50 were preincubated with different peptides at a concentration of 100 μg/ml for 1 h at 4°C. Twofold dilutions were made from this mixture with PBS containing the homologous peptide at a concentration of 10 μg/ml, and the samples were applied to the slides. Further steps were the same as for the previously described IFA (27).
In vitro neutralization assay.
The in vitro neutralization assay with the antisera raised in C57BL or C3H mice was performed as previously described (28). In brief, dilutions of sera were made in PBS containing 5% guinea pig serum, serovar E-EBs diluted in PBS were then added to the dilutions to give a final volume of 0.1 ml, and the reaction mixtures were incubated at 37°C for 45 min. Monolayers of HeLa 229 cells that had been rinsed in PBS were inoculated with 0.05 ml of the reaction mixture and were then centrifuged at 1,500 × g for 1 h at room temperature. Inoculated cell monolayers were then incubated for 1 h at 37°C, followed by the addition of 1 ml of Eagle’s minimal essential medium with Earle’s salts containing fetal bovine serum (10%), gentamicin (50 μg/ml), and cycloheximide (1 μg/ml). Cultures were then incubated for 48 h, fixed with methanol, and stained by an indirect method with a C. trachomatis species-specific monoclonal antibody (E4) and an antimouse horseradish peroxidase system (28). The results were expressed as percentages of the inclusion-forming units in control monolayers that were obtained with sera from naive C57BL and C3H mice. Neutralization was defined as a culture with <50% of control inclusion-forming units.
Lymphocyte proliferation assay.
The lymphoproliferative assay was performed as previously described (20). Briefly, the spleens from two mice from each group were harvested on day 50 after primary immunization, and the cells were teased into a single-cell suspension and enriched for T cells in a nylon wool column. Antigen-presenting cells were prepared by irradiating (3,300 rads of 137Cs) unseparated spleen cells and incubating them with the positive controls concanavalin A (Sigma, St. Louis, Mo.) and lipopolysaccharide (Sigma), medium as the negative control, test peptides, or serovar E EBs. The T-cell-enriched fraction (0.8 × 105 cells per well) and antigen-presenting cells (1.2 × 105 cells per well) were added in triplicate to 96-well U-bottom plates (Corning). Cultures were incubated for 5 days at 37°C in 5% CO2. At the end of the fourth day of incubation, 1.0 μCi of [methyl-3H]thymidine (47 Ci/mmol; Amersham, Arlington Heights, Ill.) was added to each well, and the uptake of [3H]thymidine was measured 24 h later.
Statistical analysis.
The Student unpaired t test was employed, using Statview software to compare results among the immunization groups. For statistical analysis, both optical density values from ELISA at a fixed serum dilution and antibody titers from individual mice within the different groups were compared. Unless otherwise stated, the level of significance was established at a P value of <0.05.
RESULTS
Antibody response to VD4 and EBs in immunized mice.
The antibody response to the VD4 peptide and EBs with pooled sera from mice immunized with the VD4 peptide alone are shown in Table 2. Here, with samples taken 45 days after the initial immunization, while both mouse strains were able to form antibodies to the VD4 peptide, the response in C3H mice was fourfold lower. When this anti-VD4 peptide serum was tested against EBs, only the sera from the C57 mice were able to recognize the whole organism. Vaginal wash titers to the VD4 peptide were low but detectable in both strains of mice, but antibody to EBs was not detected in the vaginal samples.
TABLE 2.
ELISA titers of antisera and vaginal wash samples to the VD4 peptide and EBsa
| Immunogen | Serum immunoglobulin
titerb in:
|
Vaginal wash IgA
titerc in:
|
||||||
|---|---|---|---|---|---|---|---|---|
| C57
mice
|
C3H mice
|
C57 mice
|
C3H
mice
|
|||||
| VD4 | EBs | VD4 | EBs | VD4 | EBs | VD4 | EBs | |
| VD4 | 64,000 | 8,000 | 16,000 | <500 | 64 | <4 | 4 | <4 |
| VD4 + hspm | 64,000 | 2,000 | 16,000 | <500 | 32 | <4 | 8 | <4 |
| VD4 + hspct | 128,000 | 16,000 | 64,000 | 8,000 | 64 | 4 | 64 | <4 |
| VD4 + TT | 128,000 | 16,000 | 32,000 | <500 | 64 | <4 | 64 | <4 |
| VD4 + pre-S2 | 128,000 | 16,000 | 8,000 | <500 | 64 | <4 | 4 | <4 |
Pooled sera were obtained 45 days after the initial immunization.
The lowest dilution tested was 1:500.
The lowest dilution tested was 1:4.
In an attempt to boost the antibody response to the EBs, in particular in C3H mice, the T-cell helper peptides shown in Table 1 were coentrapped in liposomes with the VD4 peptide and used to immunize mice. With the exception of hspct, C3H mice immunized with the T-cell helper peptides showed a modest boost in titer to both the VD4 peptide and EBs. However, hspct coadministered with the VD4 peptide to C3H mice elicited a significant fourfold rise in the serum titer to the VD4 peptide from 16,000 to 64,000 (P = 0.003), and, more importantly, antibody to the EBs rose from undetectable levels to a titer of 8,000 (P = 0.06). Thus, the addition of the hspct T-cell helper peptide elicited serum titers in C3H mice to the levels seen in C57BL6 mice immunized with only the VD4 peptide. A similar finding was obtained with vaginal wash samples.
Subclasses of antibodies to the VD4 peptide and EBs were determined to see whether the two strains of mice differed in the distribution of immunoglobulin G (IgG) subclasses in response to the VD4 peptide and EBs and whether the subclass distribution could be modified by coimmunization with the hspct helper peptide (Table 3). The two groups of C57BL mice immunized with VD4 alone or together with the hspct peptide showed only slight differences in the antibody titers elicited to both the VD4 peptide and the EBs. In addition, the distribution of subclasses with or without the helper peptide did not differ greatly. The most interesting finding with the C57 mice was the striking difference in the quantities of VD4-specific and EB-specific IgG1 that were generated. In both immunization groups, the titer to the EBs was 1,000-fold lower than the IgG1 response to the VD4 peptide. This is in contrast with all other subclasses, which showed a 2- to 16-fold difference in recognition of the VD4 peptide and the EBs. Therefore, in this strain of mice the large discrepancy seen in titers raised to the peptide versus those that recognize EBs is largely due IgG1.
TABLE 3.
Immunoglobulin subclass distribution in antisera obtained from C57 and C3H micea
| Mouse strain | Immunogen | ELISA antigen | ELISA titer
|
||||
|---|---|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | IgA | |||
| C57 | VD4 alone | VD4 | 1,024,000 | 2,000 | 256,000 | 16,000 | 16,000 |
| EBs | 4,000 | 8,000 | 16,000 | 1,000 | 1,000 | ||
| VD4 + hspct | VD4 | 1,024,000 | 8,000 | 512,000 | 32,000 | 32,000 | |
| EBs | 1,000 | 4,000 | 64,000 | <500 | 2,000 | ||
| C3H | VD4 alone | VD4 | 16,000 | 32,000 | 32,000 | 1,000 | 2,000 |
| EBs | <200 | <200 | <200 | <200 | <200 | ||
| VD4 + hspct | VD4 | 256,000 | 128,000 | 64,000 | 2,000 | 8,000 | |
| EBs | 51,200 | 12,800 | 6,400 | 1,600 | 800 | ||
Pooled sera were obtained 45 days after the initial immunization.
In contrast to the C57 mice, with the C3H strain addition of the hspct peptide both changed the subclass distribution to the VD and, as discussed above, had a significant effect on the titers to both the EBs and VD4 peptide. Addition of the T-cell helper peptide increased the antibody titer to VD4 16-fold for IgG1, 4-fold for IgG2a, and 2-fold for IgG2b. All subclasses of antibody to the EBs increased from nondetectable levels to those shown in Table 3.
Antibody response to T-cell peptides.
The ELISA titers of serum antibodies to the individual T-cell peptides, when coadministered with the VD4 peptide, are shown in Table 4. The peptide derived from murine hsp60 (hspm) was the only one that induced a significant antibody response in both strains of mice. The titer to hspm was 12,800 in C57BL mice and 1,600 in C3H mice. In contrast, the analogous region of hspct did not elicit a detectable antibody response in C57BL mice, and the antibody titer in C3H mice was 6,400. Cross-reactivities to the peptides representing the hspm and hspct T-cell epitopes were observed in both strains of mice. Here these peptides, which have 47% homology, had a titer 8- to 16-fold lower than that to the homologous hsp peptide used to immunize the mice.
TABLE 4.
ELISA titers of antisera to T-cell helper peptidesa
| Immunogen | Titer in:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| C57
mice
|
C3H mice
|
|||||||
| hspm | hspct | TT | Pre-S2 | hspm | hspct | TT | Pre-S2 | |
| VD4 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| VD4 + hspm | 12,800 | 800 | 1,600 | <100 | 1,600 | 200 | <100 | <100 |
| VD4 + hspct | <100 | <100 | <100 | <100 | 800 | 6,400 | <100 | <100 |
| VD4 + TT | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| VD4 + pre-S2 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | 3,200 |
Pooled sera were obtained 45 days after the initial immunization. The lowest dilution tested was 1:100.
As shown in Table 2, C3H mice produced antibodies to EBs only when coimmunized with the hspct peptide. Since this strain of mice also produced antibodies to the hspct peptide (Table 4), to determine whether the hspct antibodies contributed to the positive EB ELISA and IFA titers, a competitive inhibition assay was performed. Serum obtained from C3H mice immunized with coentrapped VD4 and hspct was preincubated with an excess of each peptide before being tested. The recognition of EBs by this serum could be completely blocked by the VD4 peptide but not by the hspct peptide, suggesting that antibodies to the EBs detected by ELISA or IFA were directed mainly to the VD4 region of the MOMP (data not shown).
Epitope scanning.
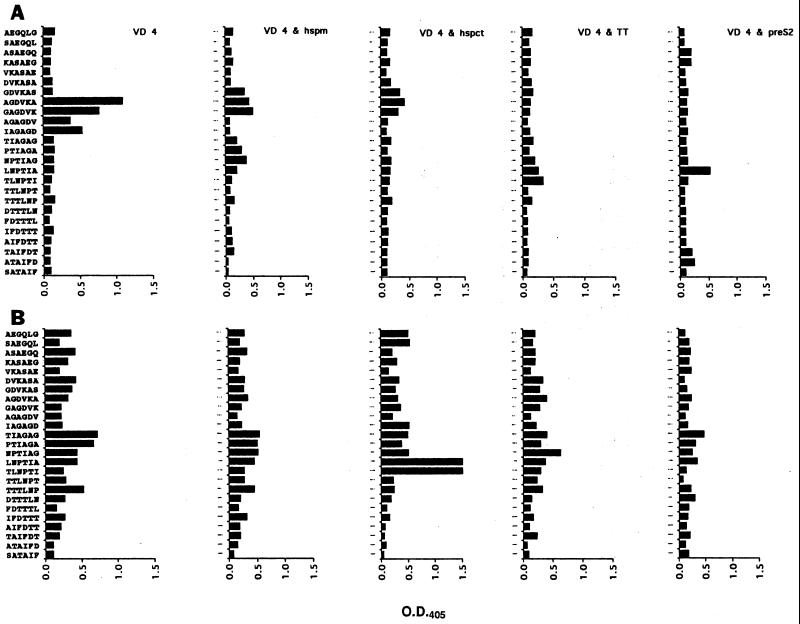
To further characterize the immune response to the VD4 peptide when coimmunized with the T-cell helper peptides, a pepscan with the immune sera was performed. C57 mice immunized with the VD4 peptide alone recognized the peptide GAGDVKA, which has previously been associated with neutralizable epitopes (Fig. 1) (23). In addition, when the hspm and pre-S2 peptides were used in the immunizations, there was modest binding of the peptide LNPTIA, which is also a neutralizing epitope (24). However, none of the T-cell helper peptides appeared to dramatically redirect or enhance the recognition of any area within VD4. With the C3H mice, with the exception of the antisera obtained from mice coimmunized with VD4 and the hspct peptides, immune sera from each group reacted to some degree with the overlapping hexameric peptides representing the VD4 sequence, but there were no dominant peptides recognized. However, the most dramatic change in the pepscan was seen when immune serum from C3H mice coimmunized with hspct and VD4 was compared to that obtained from mice immunized with VD4 alone. Here, in the presence of the hspct peptide, there was enhancement of recognition of the neutralizing epitope LNPTIAG over that with sera from the group immunized with VD4 alone.
FIG. 1.
Pepscan of the VD4 peptide with the antisera (1/200 dilutions) raised in C57BL/6 mice (A) and C3H mice (B). All antisera were pools from 7 mice bled on day 45. The peptide sequence from the bottom to the top is from the N terminus to the C terminus. O.D.405, optical density at 405 nm.
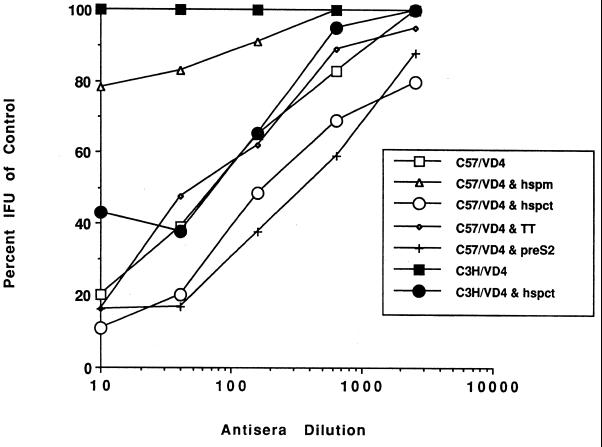
In vitro neutralization.
Antisera raised in C57BL and C3H mice to the VD4 peptide alone and to VD4 in combination with T-cell peptides were used in an in vitro neutralization assay (Fig. 2). All sera from C57BL mice, except that obtained from the group coimmunized with the VD4 and hspm peptides, were able to neutralize serovar E, with 50% neutralization titers ranging from 70, obtained when the tetanus toxin (TT) T-cell helper was used, to a high of 350, obtained with the pre-S2 T-cell helper peptide. This was in contrast with the results obtained with antisera obtained from C3H mice. Here, the only antiserum able to neutralize C. trachomatis was that obtained after coimmunization with the VD4 and hspct peptides, where the 50% neutralization titer was 75. Thus, these results correlated with the ELISA findings and showed that, regardless of the ELISA titer to the VD4 peptide, unless the titer to EBs was >8,000, no neutralization was observed (Table 2). In this regard it is interesting that with C57 mice, hspm appeared to have little effect on the ELISA titers to VD4, while the ELISA and neutralization titers to the EBs were abolished.
FIG. 2.
Pooled antisera collected on day 45 from groups of seven mice each were tested by an in vitro neutralization assay. The values shown are the averages from assays performed on separate days. The neutralization values for antisera from C3H mice coimmunized with VD4 and hspm, VD4 and TT, and VD4 and pre-S2 are not shown, since for all dilutions the values were 100% of the control values. IFU, inclusion-forming units.
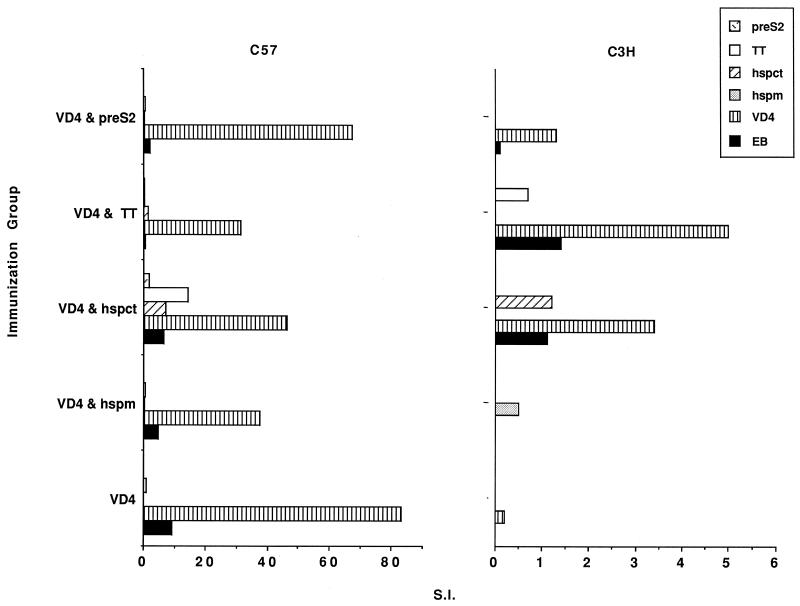
T-cell proliferative response.
To investigate whether T cells were recruited in response to the different peptides, a lymphoproliferative assay was performed with splenocytes from immunized mice (Fig. 3). All groups of immunized C57BL mice reached a significant level of proliferation in response to the VD4 peptide and were also positive, although at a lower level, to EBs. However, mice immunized with the VD4 peptide alone gave the greatest response to both the VD4 peptide and EBs. In contrast, with C3H mice a measurable lymphoproliferative response to VD4 and the EBs was seen only in the groups of mice coimmunized with the hspct or TT peptide. However, here the level of proliferation was 10 to 15 times lower than that with the C57BL mice.
FIG. 3.
Results of the lymphocyte proliferation assay, shown as the stimulation index (S.I.) for two mice in each of the indicated immunization groups. The S.I. of the control, nonimmunized mice was subtracted from each of the values. The antigen used for stimulation is indicated by the different bars. Note the different scales used for the two strains of mice.
DISCUSSION
Among the several challenges in the development of nonreplicative subunit vaccines is identifying, along with protective epitopes, T-cell epitopes that can provide help over a range of major histocompatibility complex types in humans, or H-2 phenotypes in mice. Several universal T-cell helper epitopes that have provided T-cell help for a variety of B-cell epitopes and H-2 haplotypes have been described (6, 12, 16, 19, 21). In this study, in an attempt to boost the immune response to a potential protective B-cell epitope, universal T-cell epitopes representing TT, the hepatitis B pre-S surface protein, and the mouse hsp60 were used, as well as the hsp60 region from C. trachomatis that corresponded to that of the hspm peptide (7, 12, 16, 19). The hspct peptide was used since it was reasoned that for recall upon infection with this organism, it would be advantageous if the T-cell helper was homologous to the invading pathogen. T-cell epitopes on the chlamydia MOMP and OMP-2 proteins have been described, but in this study we chose to investigate hspct because microbes as well as mammalian cells all possess an hsp60 that is expressed under stressful conditions (1, 14, 35). Upon infection of the invading pathogen, both the host and bacterial hsp60s are expressed, and thus the potential exists for both host and bacterial hsps to act as T-cell help for the invading pathogen. It was this reasoning that led Konen-Waisman et al. (16) to use hsp60 peptide homologues to provide T-cell help for otherwise T-cell-independent antigens. In addition, Zhong and Brunham (41) have shown that mice of the H-2k haplotype are capable of mounting an antibody response to the whole Chlamydia hsp60. Since one of our goals was to elicit an immune response to the VD4 peptide in the C3H (H-2k) strain, because of its susceptibility to a vaginal C. trachomatis infection, we reasoned that the hspct peptide was a good T-cell helper candidate for this strain. The use of an hsp as a chlamydial immunogen is controversial, since there is some association between antibody titers to hspct and ectopic pregnancy and infertility; however, it is still not clear whether this is due to the native host hsp60 response to the inflammation or to the contribution by the chlamydial hsp (4, 18, 22, 39). It has also been suggested that the presence of high titers to hsp60 might be related more to the length of exposure and/or severity of the chlamydial infection than to a selective response to hsp60 (4). Therefore, before considering components of this protein in a subunit vaccine, more studies on the actual role of hsp60 or epitopes of hsp60 in disease caused by a Chlamydia infection clearly are needed.
Using a peptide representing VD4 alone to immunize C57BL/10SnJ (H-2b) and seven other H-2 congenic mouse strains, Su et al. (36) reported that while strains of H-2 types b and f were able to respond with an antibody response to the VD4 peptide and whole MOMP, those of H-2 types a, d, ja, k, u, and v failed to mount an antibody response. They further showed that with a chimeric peptide incorporating both the VD4 region and the VD1 region of the MOMP, the antibody response was increased in all strains tested except those of the H-2d and H-2u haplotypes. Taking this work a step further, they immunized A/J (H-2a) mice systemically with the chimeric peptide and found a high serum but low vaginal antibody response to EBs, which was predominately IgG1. Upon challenge, however, attenuation of vaginal shedding was modest. There are several factors that could explain or contribute to this low level of protection, including the fact that the T-cell help afforded by the VD 1 peptide may have directed the response to a less protective one. Since the C57BL/6 (H-2b) strain can recognize a T-cell helper epitope within the native VD4 sequence, we also wanted to see if the VD4 peptide coadministered with other T-cell helper peptides would modify the response of this strain to the B-cell peptides within the VD4 sequence.
As determined by use of the T- and B-cell parameters examined, none of the T-cell helper peptides modified or enhanced the immune response in the C57 strain. In contrast, our data clearly showed that the hspct peptide was able to strengthen the humoral response to the VD4 peptide and EBs in the C3H strain to levels equivalent to that seen with the C57 strain, which recognizes a T-cell epitope within the VD4 sequence. However, this response as determined by use of other parameters was quite different in the two strains. First, the level of neutralization seen in the antisera from C3H mice immunized with the hspct and VD4 peptides was much lower than that in the antisera obtained from the C57 strain. The molecular specificities as judged by pepscans of the VD4 sequence were also quite different in the two mouse strains. In addition, the immunoglobulin subclass profile for EBs as well as the VD4 peptide differed in the two strains. One explanation for the difference might be that for the C57 mice, both the T- and the B-cell helper epitopes in the VD4 peptide are on the same peptide, whereas for the C3H mice, which are unable to recognize a T-cell helper epitope in the VD4 peptide, these epitopes are on separate molecules. By using in vitro systems it has been shown that epitopes on separate peptides can act together to produce T-cell bystander help for the B cells, as opposed to cognate help, which results when T- and B-cell epitopes are on the same molecule (11, 13, 32). In cognate help, where there is physical contact between the two cell types, the resulting antibodies have been shown to be of higher affinity than those produced by bystander help (32). Therefore, the lower neutralizing response exhibited by the C3H mice in this study could be due to lower-affinity antibodies being produced as a result of the physical separation of the B- and T-cell epitopes. Furthermore, it has been shown with Th1 cell clones that close physical contact is needed for differentiation of B cells due to the weak production of interleukin-5 by Th1 cell clones (13). In contrast, Th2 T-cell clones have been reported to support B-cell differentiation in the absence of contact between T and B cells (13). This has been demonstrated in vitro to be mediated in part by interleukin-5 produced in large enough quantities from the Th2 cell clones (13). This might also partially explain the different immunoglobulin profiles obtained for the two strains of mice, since different signaling events for B-cell stimulation exist with the different types of T-cell help. Therefore, while the levels of antibody might appear to be the same by ELISA and IFA in the two strains, when a separate T-cell helper peptide provided the necessary help, the response in terms of function, molecular specificity, and immunoglobulin subclass appeared to be quite different. In addition, in the C3H mice the level of lymphocyte proliferation was 10- to 15-fold less than that seen in the C57 mice. In terms of a vaccine, these differences may have profound effects on the ability of the vaccine to give protection.
To date, by using synthetic peptides of the MOMP of Chlamydia, there has been only modest protection against a genital challenge (36). The reasons for this are numerous and include the route of administration and type of immune response elicited. In this study we used a mucosal route of immunization, since we feel that this might be important for protection from a genital mucosal infection. While we achieved our goal of eliciting an immune response to the VD4 peptide in the otherwise nonresponsive C3H strain, the response in terms of neutralization and immunoglobulin profile differed from that of the responsive C57 strain. Whether this was due to a cognate versus bystander response remains to be determined. The next step in answering this question will be to compare the response of coadministered peptides to that of a chimeric peptide of the hspct and VD4 peptides used here. However, in both strains of mice the genital mucosal response as measured by Chlamydia antibody in vaginal wash specimens was low; therefore, it seems logical that immunization schemes that are able to boost the genital mucosal response are needed in order to optimize chances for successful challenge experiments, providing that the genital humoral response is an indication of the overall immune state of the animal. With regard to this, it is still not clear what in vitro parameters can predict success or failure of immunization-challenge experiments. In summary, the C3H mouse strain provides us with a good model not only in which to test the ability of different subunit vaccine candidates to elicit an immune response but also for eventual challenge experiments and subsequent determination of parameters which can predict the likelihood of successful vaccines.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AI-30499 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Allen J E, Stephens R S. An intermolecular mechanism of T cell help for the production of antibodies to the bacterial pathogen, Chlamydia trachomatis. Eur J Immunol. 1993;23:1169–1172. doi: 10.1002/eji.1830230529. [DOI] [PubMed] [Google Scholar]
- 2.Alving C R. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–446. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 3.Baehr W, Zhang Y X, Joseph T, Su H, Nano F E, Everett K D, Caldwell H D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci USA. 1988;85:4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bavoil P M, Hsia R-C, Rank R G. Prospects for a vaccine against Chlamydia genital disease. I. Microbiology and pathogenesis. Bull Inst Pasteur. 1996;94:5–54. [Google Scholar]
- 5.Caldwell H D, Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982;35:1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celis E, Ou D, Otvos L., Jr Recognition of hepatitis B surface antigen by human T lymphocytes. Proliferative and cytotoxic responses to a major antigenic determinant defined by synthetic peptides. J Immunol. 1988;140:1808–1815. [PubMed] [Google Scholar]
- 7.Cerrone M C, Ma J J, Stephens R S. Cloning and sequence of the gene for heat shock protein 60 from Chlamydia trachomatis and immunological reactivity of the protein. Infect Immun. 1991;59:79–90. doi: 10.1128/iai.59.1.79-90.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darville T, Andrews C W, Jr, Laffoon K K, Shymasani W, Kishen L R, Rank R G. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Maza L M, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geysen H M, Rodda S J, Mason T J, Tribbick G, Schoofs P G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987;102:259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- 11.Gregoriadis G, Wang Z, Barenholz Y, Francis M J. Liposome-entrapped T-cell peptide provides help for a co-entrapped B-cell peptide to overcome genetic restriction in mice and induce immunological memory. Immunology. 1993;80:535–540. [PMC free article] [PubMed] [Google Scholar]
- 12.Ho P C, Mutch D A, Winkel K D, Saul A J, Jones G L, Doran T J, Rzepczyk C M. Identification of two promiscuous T cell epitopes from tetanus toxin. Eur J Immunol. 1990;20:477–483. doi: 10.1002/eji.1830200304. [DOI] [PubMed] [Google Scholar]
- 13.Julius M, Haughn L. The induction of resting B cell differentiation does not require T cell contact. Eur J Immunol. 1992;22:2323–2329. doi: 10.1002/eji.1830220922. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann S H, Schoel B, van Embden J D, Koga T, Wand-Wurttenberger A, Munk M E, Steinhoff U. Heat-shock protein 60: implications for pathogenesis of and protection against bacterial infections. Immunol Rev. 1991;121:67–90. doi: 10.1111/j.1600-065x.1991.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 15.Kirby C, Gregoriadis G. Dehydration-rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Biotechnology. 1984;2:979–984. [Google Scholar]
- 16.Konen-Waisman S, Fridkin M, Cohen I R. Self and foreign 60-kilodalton heat shock protein T cell epitope peptides serve as immunogenic carriers for a T cell-independent sugar antigen. J Immunol. 1995;154:5977–5985. [PubMed] [Google Scholar]
- 17.Morrison R P, Manning D S, Caldwell H D. Immunology of Chlamydia trachomatis infection. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press; 1992. pp. 57–84. [Google Scholar]
- 18.Morrison R P, Su H, Lyng K, Yuan Y. The Chlamydia trachomatis hyp operon is homologous to the groE stress response operon of Escherichia coli. Infect Immun. 1990;58:2701–2705. doi: 10.1128/iai.58.8.2701-2705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neurath A R, Kent S B, Strick N, Parker K, Courouce A M, Riottot M M, Petit M A, Budkowska A, Girard M, Pillot J. Antibodies to synthetic peptides from the pre-S1 and pre-S2 regions of one subtype of the hepatitis B virus (HBV) envelope protein recognize all HBV subtypes. Mol Immunol. 1987;24:975–980. doi: 10.1016/0161-5890(87)90009-5. [DOI] [PubMed] [Google Scholar]
- 20.Pal S, Fielder T J, Peterson E M, de la Maza L M. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 22.Peeling R W, Bailey R L, Conway D J, Holland M J, Campbell A E, Jallow O, Whittle H C, Mabey D C. Antibody response to the 60-kDa chlamydial heat-shock protein is associated with scarring trachoma. J Infect Dis. 1998;177:256–259. doi: 10.1086/517367. [DOI] [PubMed] [Google Scholar]
- 23.Peterson, E. M., and X. Cheng. Unpublished data.
- 24.Peterson E M, Cheng X, Markoff B A, Fielder T J, de la Maza L M. Functional and structural mapping of Chlamydia trachomatis species-specific major outer membrane protein epitopes by use of neutralizing monoclonal antibodies. Infect Immun. 1991;59:4147–4153. doi: 10.1128/iai.59.11.4147-4153.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson E M, Cheng X, Pal S, de la Maza L M. Effects of antibody isotype and host cell type on in vitro neutralization of Chlamydia trachomatis. Infect Immun. 1993;61:498–503. doi: 10.1128/iai.61.2.498-503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Peterson E M, Markoff B A, de la Maza L M. The major outer membrane protein nucleotide sequence of Chlamydia trachomatis, serovar E. Nucleic Acid Res. 1990;18:3414. doi: 10.1093/nar/18.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson E M, Motin V L, You J, de la Maza L M. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. 1998. Protection of mice from an intravaginal challenge with Chlamydia trachomatis, serovar E, by intranasal immunization with serovar E; pp. 454–457. [Google Scholar]
- 27.Peterson E M, Oda R, Tse P, Gastaldi C, Stone S C, de la Maza L M. Comparison of a single-antigen microimmunofluorescence assay and inclusion fluorescent-antibody assay for detecting chlamydial antibodies and correlation of the results with neutralizing ability. J Clin Microbiol. 1989;27:350–352. doi: 10.1128/jcm.27.2.350-352.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson E M, Zhong G M, Carlson E, de la Maza L M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988;56:885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu Z, Cheng X, de la Maza L M, Peterson E M. Analysis of the humoral response elicited in mice by a chimeric peptide representing variable segments I and IV of the major outer membrane protein of Chlamydia trachomatis. Vaccine. 1994;12:557–564. doi: 10.1016/0264-410x(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 30.Qu Z, Cheng X, de la Maza L M, Peterson E M. Characterization of a neutralizing monoclonal antibody directed at variable domain I of the major outer membrane protein of Chlamydia trachomatis C-complex serovars. Infect Immun. 1993;61:1365–1370. doi: 10.1128/iai.61.4.1365-1370.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schachter J. Overview of Chlamydia trachomatis infection and the requirements for a vaccine. Rev Infect Dis. 1985;7:713–716. doi: 10.1093/clinids/7.6.713. [DOI] [PubMed] [Google Scholar]
- 32.Shaw D M, Stanley C M, Partidos C D, Steward M W. Influence of the T-helper epitope on the titre and affinity of antibodies to B-cell epitopes after co-immunization. Mol Immunol. 1993;30:961–968. doi: 10.1016/0161-5890(93)90121-q. [DOI] [PubMed] [Google Scholar]
- 33.Stephens R S, Sanchez-Pescador R, Wagar E A, Inouye C, Urdea M S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987;169:3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su H, Caldwell H D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992;175:227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H, Morrison R P, Watkins N G, Caldwell H D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990;172:203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su H, Parnell M, Caldwell H D. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against chlamydial genital tract infection. Vaccine. 1995;13:1023–1032. doi: 10.1016/0264-410x(95)00017-u. [DOI] [PubMed] [Google Scholar]
- 37.Taylor-Robinson D, Ward M E. Immunity to chlamydial infection and the outlook for vaccination. In: Meheus A, Spier R E, editors. Vaccines for sexually transmitted diseases. London, United Kingdom: Butterworths; 1989. pp. 67–85. [Google Scholar]
- 38.Tuffrey M, Alexander F, Conlan W, Woods C, Ward M. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J Gen Microbiol. 1992;138:1707–1715. doi: 10.1099/00221287-138-8-1707. [DOI] [PubMed] [Google Scholar]
- 39.Wagar E A, Schachter J, Bavoil P, Stephens R S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990;162:922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y X, Stewart S J, Caldwell H D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989;57:636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong G, Brunham R C. Antibody responses to the chlamydial heat shock proteins hsp60 and hsp70 are H-2 linked. Infect Immun. 1992;60:3143–3149. doi: 10.1128/iai.60.8.3143-3149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]