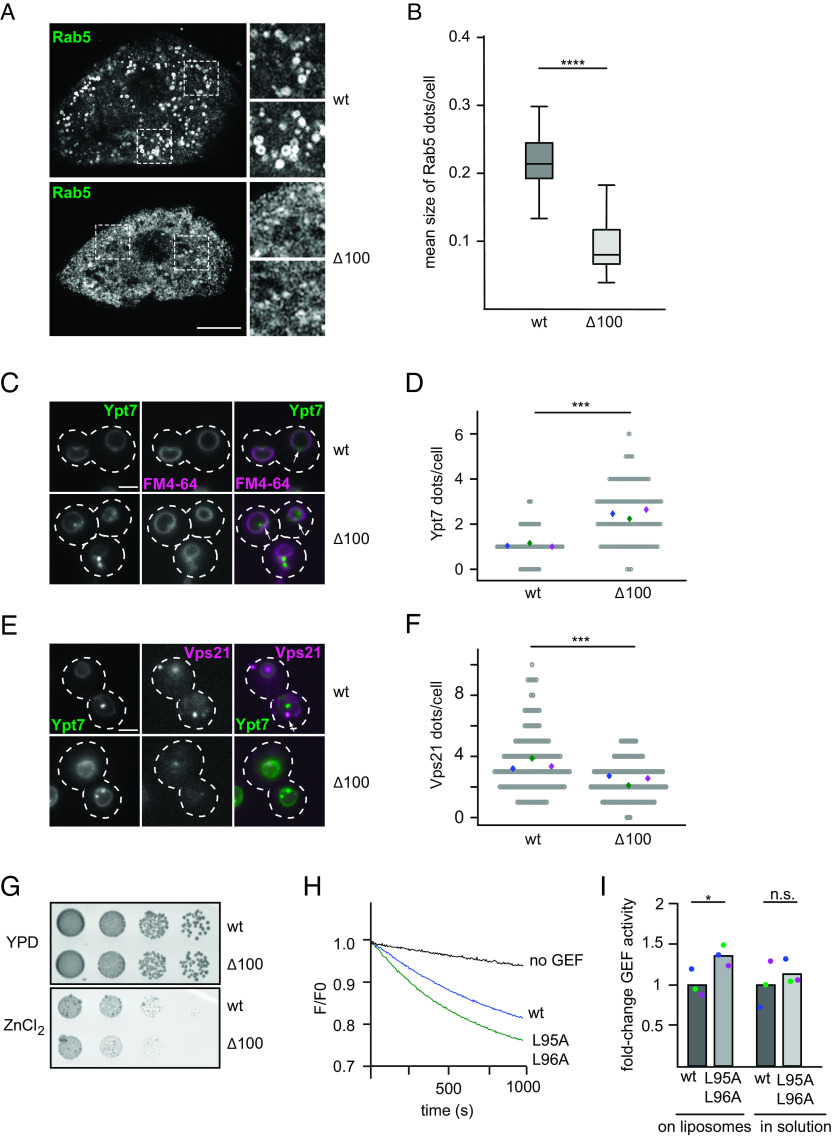
Fig. 2.
Loss of the disordered Mon1 N-terminal region affects localization of endosomal Rab GTPases and Rab5-dependent GEF activity. (A) Localization of endogenous Rab5 in Drosophila nephrocytes from 3rd instar larvae expressing wild type or Mon1∆100 under the control of the handC-GAL4 driver. Antibodies against Rab5 were used. Optical sections show the distribution of Rab5 in detail. Size bar: 10 µm. (B) Analysis of the mean size of Rab5 dots per cell from 15 cells from three animals for wild-type Mon1 and 13 cells from four animals for Mon1∆100. (P value ****< 0.0001 using an unpaired, two-sample Student’s t test). (C–F) Truncation of the Mon1 N-terminal affects Ypt7 and Vps21 localization. (C) Localization of mNEON-Ypt7 under its endogenous promotor in yeast cells in the presence of endogenously expressed wild type or Mon1Δ100 using fluorescence microscopy. Vacuoles were stained with FM4-64. Size bar, 2 µm, arrows show representative Ypt7 accumulations. (D) Quantification of Ypt7-positive dots in C. Cells (n ≥ 50) were counted from three independent experiments; (P value ***< 0.001 using a two-sample Student’s t test assuming equal variances). For image processing details, see the Materials and Methods section. (E) Localization of mCherry-tagged Vps21 in cells expressing mNeon-Ypt7 and endogenously expressed wild type or Mon1Δ100 using fluorescence microscopy. Size bar 2 µm, arrows show representative Vps21 accumulations. (F) Quantification of Vps21-positive dots in E. Cells (n ≥ 50) were counted from three independent experiments. (P value ***< 0.001 using a two-sample Student’s t test assuming equal variances). For image processing details, see the Materials and Methods section. (G) Effect on cell growth by Mon1 truncations. Strains endogenously expressing wild type or Mon1Δ100 were grown to the same OD600 in Yeast extract peptone dextrose (YPD) media and spotted in serial dilutions onto agar plates containing YPD or YPD supplemented with 4 mM ZnCl2. Plates were incubated for several days at 30 °C. (H) A hydrophobic patch mutation in the N-terminal part of Mon1 affects GEF activity. Liposomes were loaded with 150 nM prenylated Ypt10 in the presence of 200 µM GTP and 1.5 mM EDTA. The nucleotide was stabilized using 3 mM MgCl2. 250 nM Mant-GDP-loaded Ypt7:GDI was added, and the reaction was triggered by adding 12.5 nM wild-type (blue) or mutant complex expressing Mon1L95A,L96A (green). The decrease in fluorescence detected over time is normalized to initial fluorescence. (I) Comparison of fold-change in GEF activity of wild-type and Mon1L95A,L96A mutant GEF complexes on liposomes and in solution. For details of in solution GEF assay, see the Materials and Methods section. For the GEF assay on liposomes, kobs of each curve was determined as described in the Materials and Methods section. kobs values were normalized to the wild-type value. For in solution assays, kcat/Km values were determined and normalized to the wild-type value. Bar graphs represent average fold-change, and dots represent individual changes from three experiments. (P value: *P < 0.05 using a two-sample Student’s t test assuming equal variances).