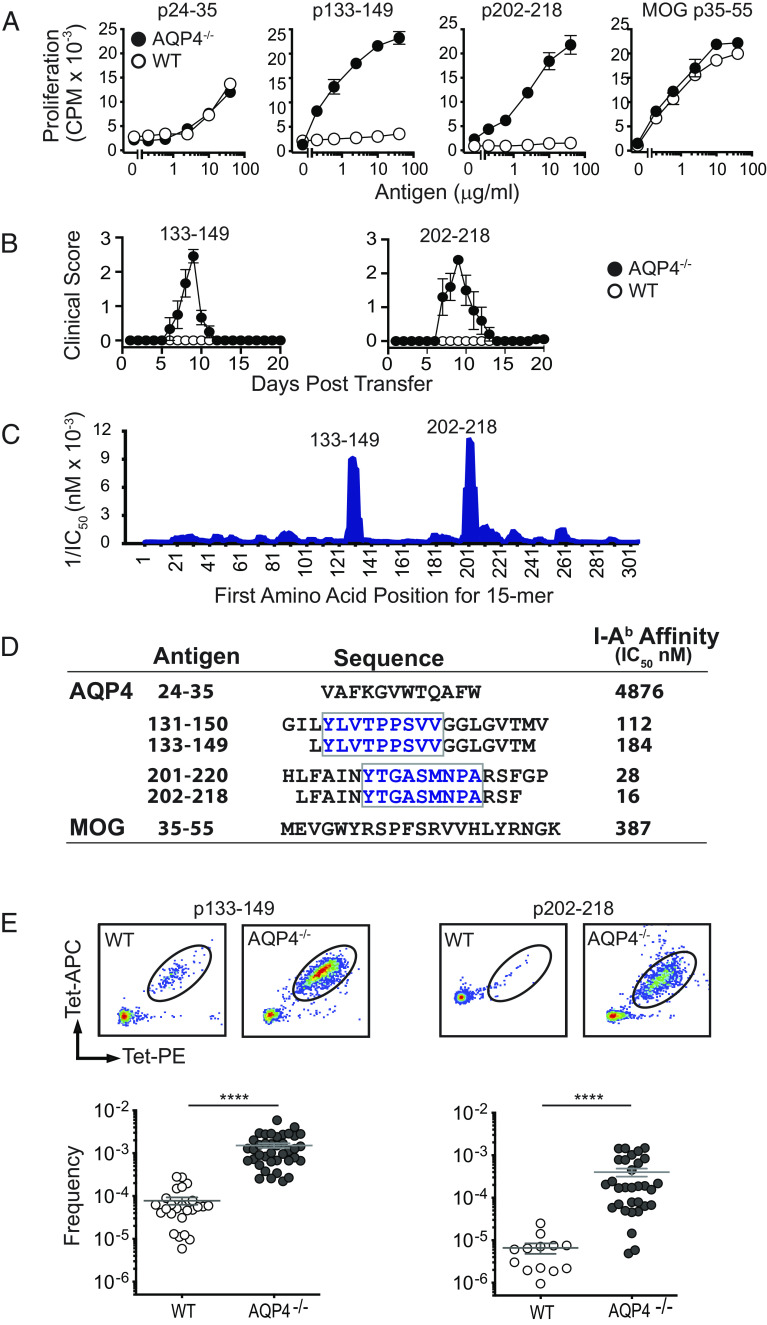
Fig. 2.
Pathogenic AQP4 determinants bind I-Ab with high affinity and elicit proliferation of AQP4:I-Ab-specific TCR-bearing T cells in AQP4-deficient mice. (A) WT or AQP4−/− mice were immunized s.c. with peptides indicated. Peptide-specific proliferation of draining lymph node cells was measured by 3H-thymidine incorporation (mean ± SEM, representative of five experiments). (B) Lymph node cells from AQP4 immunized WT or AQP4−/− mice were cultured for 3 d with cognate antigen under Th17 polarizing conditions. AQP4 p133–149- and p202–218-specific Th17 cells from AQP4−/− donors induce paralysis in WT recipient mice. (C) IEDB, an in silico method for predicting determinants within proteins that bind MHC alleles, was used to identify AQP4 amino acid sequences anticipated to bind I-Ab for recognition by AQP4-specific/I-Ab-restricted CD4+ T cells. 1/IC50 is plotted against the first amino acid for each overlapping AQP4 15 mer. Peaks correspond to increased predicted binding affinity. (D) Purified I-Ab molecules were tested biochemically for peptide binding affinity using a competitive binding assay between a radiolabeled high-affinity competitor peptide and unlabeled peptides (21). Lower IC50 values indicate higher binding affinity. Boxed amino acids designate the core binding regions. (E) Lymph node T cells isolated from WT or AQP4−/− mice immunized with AQP4 p133–149 or p202–218 were stained with I-Ab:p133–149 and I-Ab:p220–218 tetramers, bead-enriched, and analyzed by flow cytometry. Upper panels show representative tetramer staining of bead-enriched lymph node cell samples. Lower panels show frequency of tetramer-binding T cells among CD4+ T cells, calculated from enriched and unenriched fractions using counting beads for quantification. Each data point represents one mouse. ****P < 0.0001 (Mann–Whitney U test).