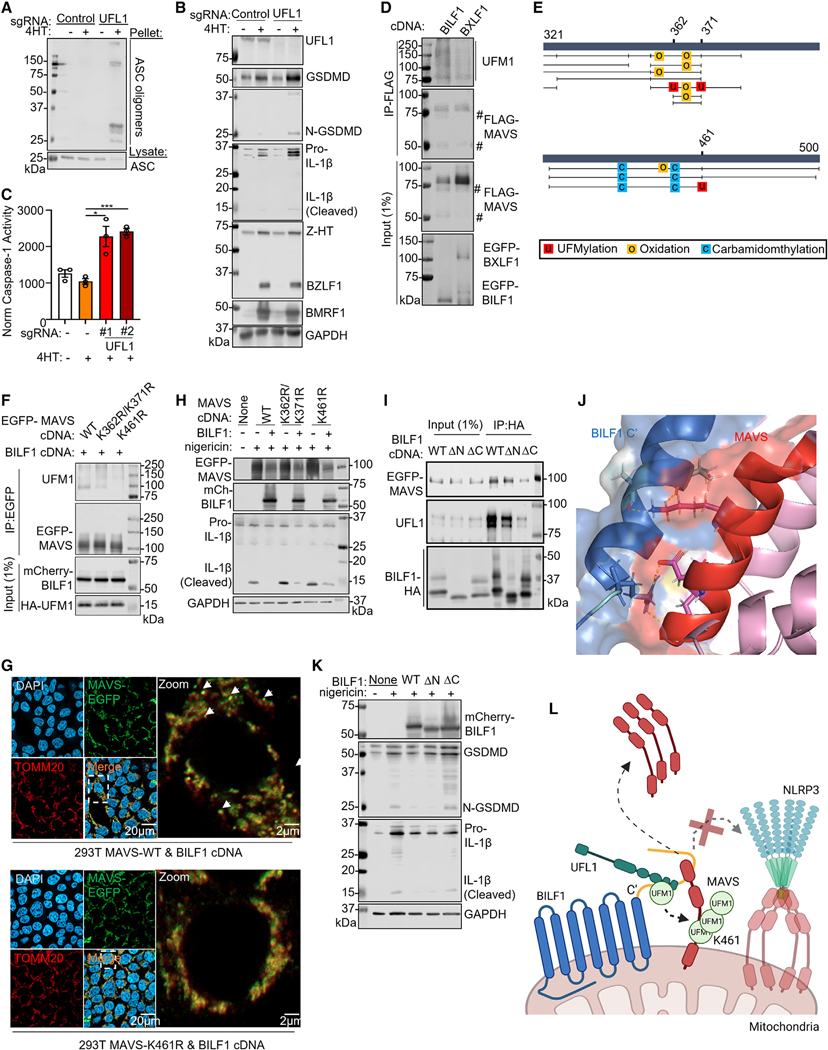
Figure 5. BILF1 triggers MAVS mitochondrial dislocation through UFMylation.
(A) Immunoblot of ASC oligomerization from P3HR-1 expressing the indicated sgRNA, uninduced or 4HT-induced for 24 h.
(B) Immunoblot analysis of WCL from P3HR-1 expressing the indicated sgRNA and 4HT-induced, as indicated.
(C) Mean ± SEM caspase-1 activity normalized by live cell number from n = 3 replicates of P3HR-1 expressing the indicated sgRNA and 4HT-treated for 24 h, as indicated.
(D) Immunoblot of 1% input and anti-FLAG-MAVS complexes from 293T co-transfected with FLAG-MAVS, BILF1, or BXLF1 cDNAs for 24 h, as indicated.
(E) PEAKS software identification of potential MAVS post-translational modification sites. Putative UFMylation sites were identified at lysines 362, 371, and 461. MAVS residues 321–500 are depicted. Black vertical lines represent individual peptide sequencing events.
(F) Immunoblot of anti-EGFP-MAVS immunopurified from 293T co-transfected with the indicated MAVS, BILF1, and UFM1 cDNAs for 24 h.
(G) Immunofluorescence analysis of wild type or K461R MAVS subcellular localization in 293T co-transfected with MAVS and BILF1 cDNAs for 24 h.
(H) Immunoblot of WCL from 293T expressing MAVS and BILF1 cDNA, nigericin stimulated for 24 h, as indicated.
(I) Immunoblot of 1% input and anti-HA immunopurified EGFP-MAVS and UFL1 from 293T transfected with BILF1 and EGFP-MAVS cDNAs for 24 h, as indicated.
(J) AlphaFold multimer model highlighting the predicted BILF1 and MAVS interaction domain and residues.
(K) Immunoblot of WCL from 293T expressing wild type, N- (ΔN) or C- (ΔC) terminal tail deletion mutant BILF1, nigericin stimulated for 24 h, as indicated.
(L) Schematic of BILF1 NLRP3 inflammasome inhibition.
Student’s t test was performed, with ***p < 0.001. *p < 0.05. See also Figures S4 and S5. Immunoblots are representative of n = 2.