Table 1.
Selective Aurora-A inhibitor alisertib in clinical trailsa.
| Drug | Structure | Target | Type of cancer | Phase/Status | Design | Clinical trial numberb |
|---|---|---|---|---|---|---|
| Alisertib (MLN8237) | 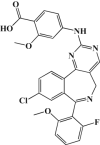 |
Aurora-A IC50 1.2 nmol/L |
Relapsed/refractory peripheral T-cell lymphoma | III/C | M | NCT01482962 |
| Aggressive non-Hodgkin's lymphoma; Acute myelogenous leukemia and high-grade myelodysplastic syndrome; Children with recurrent/refractory solid tumors and leukemias; Ovarian carcinoma; Recurrent or persistent leiomyosarcoma of the uterus; Metastatic castrate resistant and neuroendocrine prostate cancer; Advanced or metastatic sarcoma; Rhabdoid tumors |
II/C | M | NCT00807495; NCT00830518; NCT01154816; NCT00853307; NCT01637961; NCT01799278; NCT01653028; NCT02114229 | |||
| Unresectable Stage III–IV Melanoma | II/T (low accrual) | M | NCT01316692 | |||
| Malignant mesothelioma | II/NR | M | NCT02293005 | |||
| Unspecified childhood solid tumor, excluding CNS neuroblastoma | I/C | M | NCT02444884 | |||
| Myelofibrosis or relapsed or refractory acute megakaryoblastic leukemia | NR | M | NCT02530619 | |||
| Chemotherapy-pretreated urothelial cancer | II/C | M or CW: Paclitaxel | NCT02109328 | |||
| Relapsed or refractory B-cell non-Hodgkin lymphoma | II/C | M or CW: Rituximab | NCT01812005 | |||
| Locally advanced or metastatic, endocrine-resistant breast cancer | II/NR | M or CW: Fulvestrant | NCT02860000 | |||
| High-risk AML | II/C | CW: Induction chemotherapy | NCT02560025 | |||
| Small cell lung cancer | II/C | CW: Paclitaxel | NCT02038647 | |||
| Metastatic or locally recurrent breast cancer | II, NR | CW: Paclitaxel | NCT02187991 | |||
| Refractory multiple myeloma | I, II/C | CW: Bortezomib | NCT01034553 | |||
| Relapsed or refractory aggressive B-cell lymphoma | I, II/C | CW: Rituximab and vincristine | NCT01397825 | |||
| Non-small cell lung cancer | I, II/C | CW: Erlotinib | NCT01471964 | |||
| Neuroblastoma | I, II/C | CW: Irinotecan and temozolomide | NCT01601535 | |||
| Hormone-resistant prostate cancer | I, II/C | CW: Abiraterone and prednisone | NCT01848067 | |||
| Rb-deficient head and neck squamous cell cancer | I, II/R | CW: Pembrolizumab | NCT04555837 | |||
| Relapsed or recurrent Hodgkin lymphoma, B-Cell non-Hodgkin lymphoma, or peripheral T-cell lymphoma | I/C | CW: Vorinostat | NCT01567709 | |||
| Relapsed or refractory B-Cell or T-Cell lymphomas | I/C | CW: Romidepsin | NCT01897012 | |||
| Metastatic breast cancer | I/C | CW: MLN0128c | NCT02719691 | |||
| Metastatic EGFR-mutant lung cancer | I/C | CW: Osimertinib | NCT04085315 | |||
| Recurrent high grade gliomas | I/C | CW: Hyperfractionated radiation therapy | NCT02186509 | |||
| Head and neck cancer | I/C | CW: Cetuximab and definitive Radiation | NCT01540682 | |||
| Pancreatic cancer | I/C | CW: Gemcitabine | NCT01924260 | |||
| Gastrointestinal tumors | I/C | CW: mFOLFOX | NCT02319018 | |||
| Colorectal cancer | I/C | CW: Irinotecan | NCT01923337 | |||
| Solid tumors | I/C | CW: Pazopanib | NCT01639911 | |||
| Relapsed and refractory mantle cell and low grade non-Hodgkin lymphoma | I, NR | CW: Bortezomib and rituximab | NCT01695941 |
Abbreviations: C, completed, M, monotherapy; CW, combination with; NR, not recruiting; R, recruiting; T, terminated; mFOLFOX: a modified oxaliplatin, fluorouracil, and leucovorin regimen.
www.clinicaltrials.gov (accessed on March 2022).
MLN0128: a dual TORC1/2 inhibitor.
