Figure 1.
Polar flagellum model in V. alginolyticus
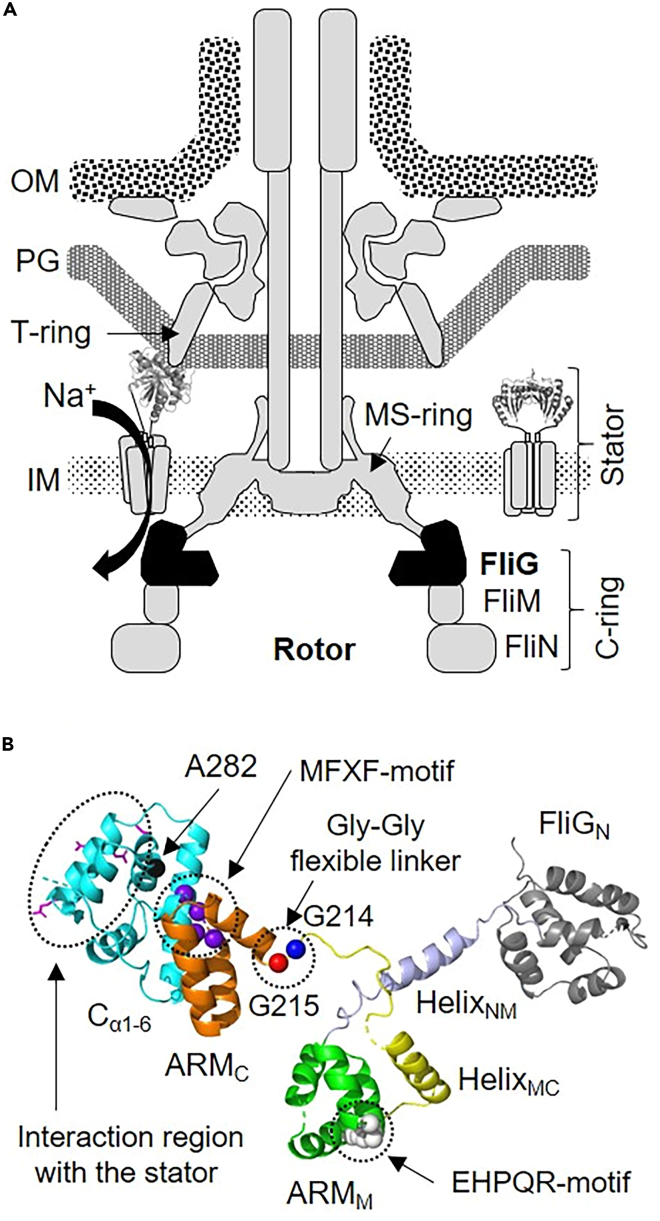
(A) Model of the rotary motor in the polar flagellum of Vibrio alginolyticus.
The C-ring is composed of three proteins; FliG, FliM and FliN. The inactive stator complex (stator in right side of the motor) diffuses in the inner membrane (IM). When the stator activates around the rotor (stator on the left side of the motor), it interacts with FliG to generate torque due to ion flex in the channel pore. Then, the C-terminal region of PomB (PomBC) binds to the curvature of the peptidoglycan layer (PG) of the T-ring. This binding allows the stator to remain around the rotor and undergo continued activation. OM represents the outer membrane.
(B) Structural model of V. alginolyticus FliG. FliG comprises three domains, N-terminal (FliGN), middle (FliGM), and C-terminal (FliGC). The latter two contain ARMM and helixMC and ARMC and Cα1-6, respectively. HelixNM connects FliGN with FliGM. The structure of FliGN, HelixNM contained N- and C-terminal loop, ARMM HelixMC contained N- and C-terminal loop, ARMC, and Cα1-6 are shown in gray, light blue, green, yellow, orange, and cyan, respectively, as a ribbon model. The atoms of white, blue, red, purple, and black balls show EHPQR motif, Gly-Gly flexible linker region (G214 and G215 residues), MFXF-motif and A282 residue. Side chains of highly conserved charged residues interacting with the stator are shown in magenta as a stick model.