Figure 3. Vaccine-driven antigen spreading is required for long-tumor tumor control in immunocompetent mice.
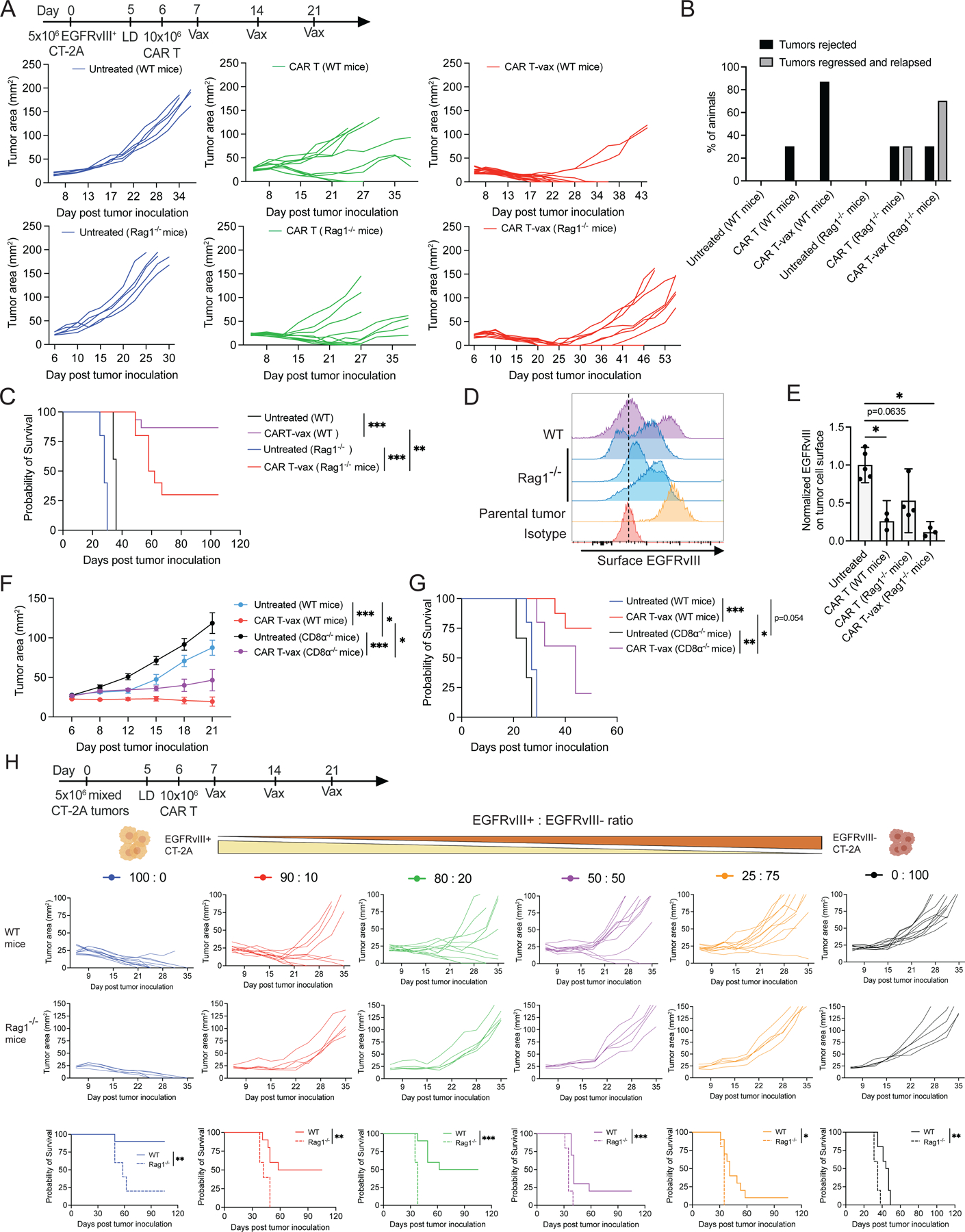
(A-E) Treatment of tumor-bearing WT or Rag1−/− mice with WT CAR-T ± vax.
(A) Tumor growth in individual mice. Untreated, n = 5; CAR-T in WT mice, n = 10; CAR T-vax, n = 15 and 10 in WT and Rag1−/− mice, respectively.
(B) Percentage of mice that completely rejected tumors or experienced tumor relapse.
(C) Overall survival.
(D-E) Surface EGFRvIII expression (D) and mean expression normalized to untreated tumors (E) on parental or representative relapsed tumors from WT and Rag1−/− mice following CAR T-vax treatment.
(F-G) Tumor-bearing WT or CD8α−/− mice (n=5–8) ± CAR T-vax treatment.
(F) Tumor growth.
(G) Overall survival.
(H) Individual tumor growth and overall survival of WT (n=10) or Rag1−/− mice (n=5) bearing heterogeneous CT-2A tumors upon CAR T-vax treatment. EGFRvIII+:EGFRvIII− cells were pre-mixed at the indicated ratios.
All mice in A-G bear EGFRvIII+CT-2A tumors. Error bars are mean ± 95% CI, ***, p<0.0001; **, p<0.01; *, p<0.05 by Student’s t-test for E, by Log-rank (Mantel-Cox) test for C,G-H, by two-way ANOVA with Tukey’s post-test for F.
