Figure. 5. Enhanced IFN-γ production by vaccine-boosted CAR T-cells is critical for antigen spreading.
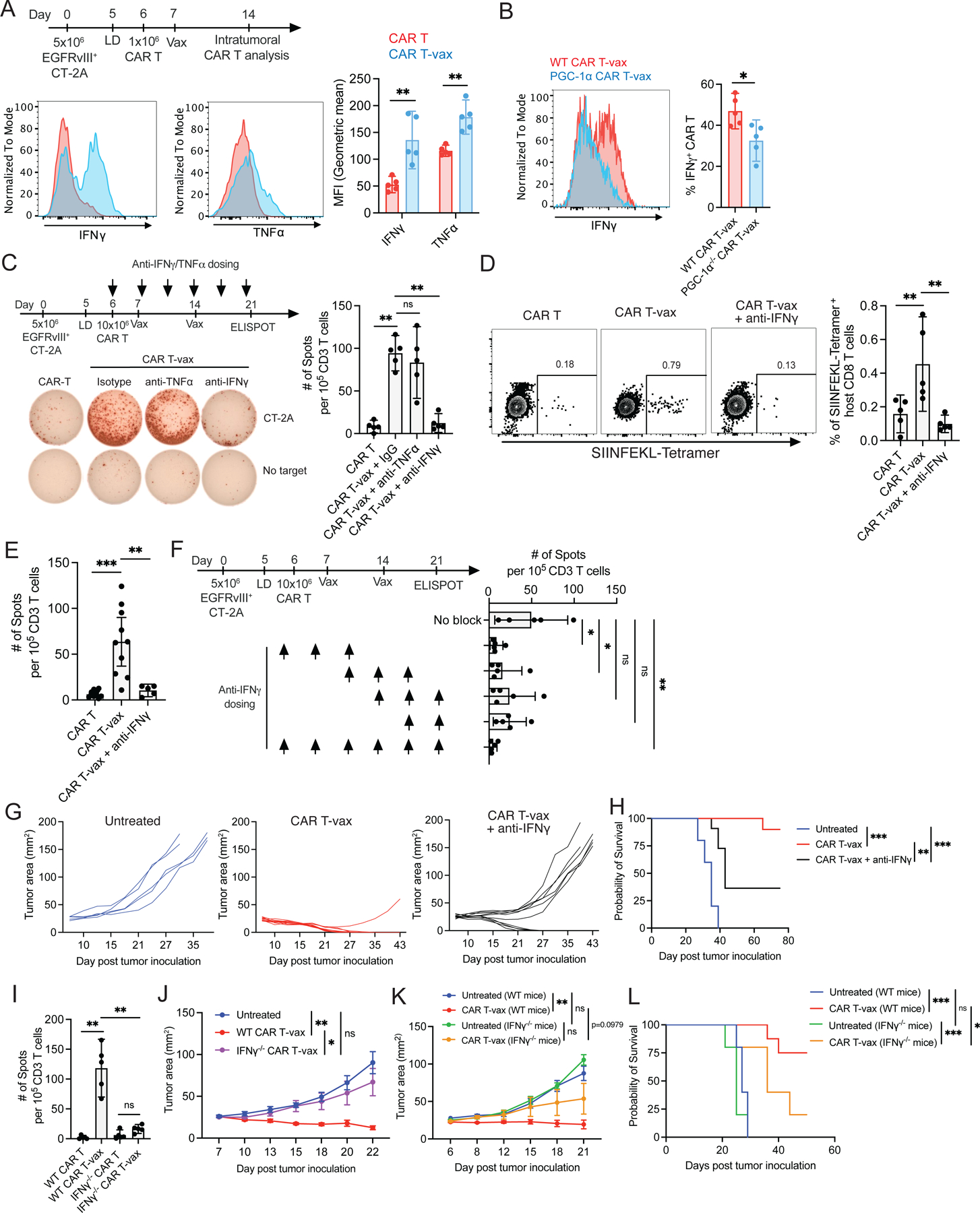
(A) IFN-γ and TNF-α expression in intratumoral CAR T-cells from mice (n=5) treated with WT CAR T ± vax.
(B) IFN-γ expression in intratumoral CAR T-cells from mice (n=5) 7 days post treatment with WT or PGC-1α−/− CAR T-vax.
(C) IFN-γ ELISPOT. Tumor-bearing mice (n=5) treated with WT CAR T or WT CAR T-vax + isotype control antibody (IgG), anti-TNF-α or anti-IFN-γ.
(D-E) OVA+EGFRvIII+CT-2A tumor-bearing mice(n=5–10) treated by WT CAR T or WT CAR T-vax ± anti-IFN-γ. Endogenous OVA-specific T-cell responses detected by SIINFEKL-tetramer staining (D) and IFN-γ ELISPOT (E).
(F) IFN-γ ELISPOT. Tumor-bearing mice (n=5) treated with WT CAR T-vax ± anti-IFN-γ at indicated time points.
(G-H) Tumor growth (G) and overall survival (H) of mice left untreated (n=5) or treated (n=10) with WT CAR T-vax ± anti-IFN-γ.
(I) IFN-γ ELISPOT. Tumor-bearing mice (n=5) treated with WT or IFN-γ−/− CAR T ± vax.
(J) Tumor growth in mice (n=5) left untreated or treated with WT or IFN-γ−/− CAR T-vax.
(K-L) Tumor growth (K) and overall survival (L) of WT or IFN-γ−/− mice (n=5–8) treated with or without WT CAR T-vax therapy.
All mice bear EGFRvIII+CT-2A tumors. Error bars are mean ± 95% CI, ***, p<0.0001; **p<0.01; *, p<0.05, ns, not significant by Student’s t-test for A-B, by one-way ANOVA with Tukey’s post-test for C-F, I, by two-way ANOVA with Tukey’s post-test for J-K, and by Log-rank (Mantel-Cox) test for H and L.
