Figure. 7. Engineering CAR T-cells for increased IFN-γ expression synergizes with vaccine boosting to enhance antigen spreading and rejection of solid tumors with pre-existing antigen heterogeneity.
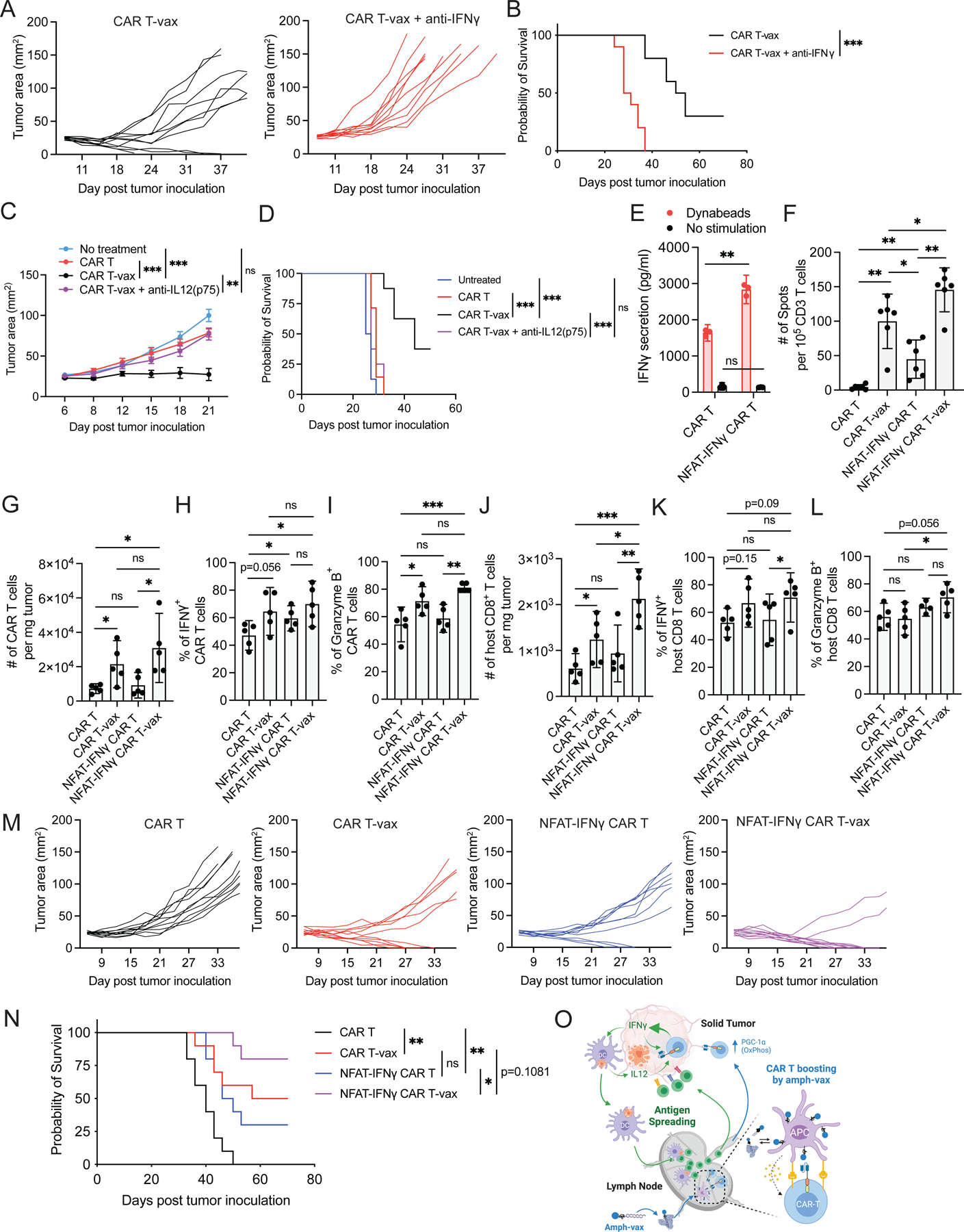
(A-D). Heterogenous CT-2A tumors were established in C57BL/6 mice.
(A)Tumor growth and (B)survival of mice (n=10) after treatment with WT CAR T-vax therapy ± anti-IFN-γ.
(C)Tumor growth and (D)survival of mice (n=8) left untreated, receiving WT CAR T, or WT CAR T-vax ± anti-IL12 (p75).
(E) IFN-γ secretion from WT or NFAT-IFN-γ CAR T-cells ± anti-CD3/CD28 beads (n=3).
(F) IFN-γ ELISPOT. EGFRvIII+ CT-2A tumor-bearing mice (n=6) treated with WT or NFAT-IFN-γ CAR T ± vax.
(G-L) Mice bearing heterogenous CT-2A tumors (n=5) treated with WT or NFAT-IFN-γ CAR T ± vax therapy. Enumeration of CAR T (G) and endogenous CD8+ T cells (J) infiltrated into tumors as well as the expression of IFN-γ (H for CAR T, K for host CD8 T) and granzyme B (I for CAR T, L for host CD8 T).
(M-N) Tumor growth (M) and overall survival (N) of mice bearing heterogenous CT-2A tumors (n=10) treated with WT or NFAT-IFN-γ CAR T ± vax.
(O) Schematic overview of CAR T-vax therapy triggered antigen spreading. Created with BioRender.com.
Heterogenous CT-2A tumors are EGFRvIII+:EGFRvIII− cells mixed at 80:20 ratio. Error bars are mean ± 95% CI. ***, p<0.001; **, p<0.01; *, p<0.05; ns not significant by one-way ANOVA with Tukey’s post-test for E-L, by two-way ANOVA with Tukey’s post-test for C, and Log-rank (Mantel-Cox) test for B, D and N.
