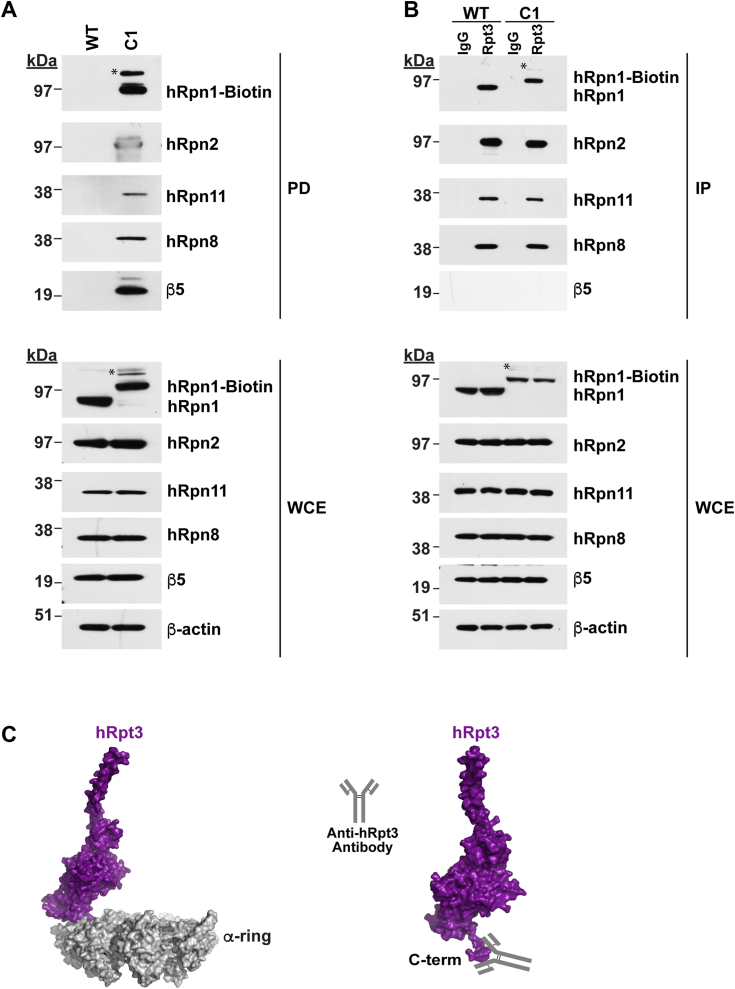
Figure 4.
26S proteasomes are isolated from C1 cells by using the biotin tag on hRpn1.A, pull-down of hRpn1-biotin from the C1 cell line by streptavidin magnetic beads. The experiment was performed in parallel with parental WT as a negative control. Proteins from the streptavidin-bound samples were eluted from the beads by SDS buffer and heating (pull-down, top) and, along with the whole cell extract (WCE, bottom), were immunoprobed for hRpn1, hRpn2, hRpn11, hRpn8, and β5. B, immunoprecipitation of 26S proteasomes from WT or C1 cells by hRpt3 or IgG (as a control) antibodies. The immunoprecipitants were dissolved in SDS buffer and heated and, along with WCE, immunoprobed for hRpn1, hRpn2, hRpn11, hRpn8, and β5. β-actin was used as a loading control. An asterisk symbol (∗) in (A) and (B) indicates a nonspecific higher molecular weight band above hRpn1-biotin. C, surface view of the 26S proteasome showing only hRpt3 (purple) with the antibody-binding site indicated (right) or also with the CP α-ring (gray, left) to illustrate the entrapment of the antibody-binding site within the CP α-ring. PDB 5GJQ was used to generate this figure. CP, core particle; PDB, Protein Data Bank.