Abstract
BACKGROUND:
Primary hyperoxaluria (PH) is a family of rare, life-threatening genetic liver disorders characterized by elevated production and excretion of oxalate. To date, the clinical and economic burden associated with PH has not been well characterized due to the rarity of the disease and previous challenges with diagnostic coding that prevented proper identification of patients with PH in claims data.
OBJECTIVE:
To characterize the clinical and economic costs, as well as health care resource utilization (HCRU), associated with PH relative to a matched cohort of patients without PH.
METHODS:
Data from the IQVIA PharMetrics Plus Database were used to conduct a retrospective matched-cohort study to compare differences in clinical characteristics, HCRU, and pharmacy and medical costs in patients with PH compared with a matched cohort of patients without PH from January 2014 to December 2019.
RESULTS:
Overall, 324 patients were included in the PH cohort and 1,620 patients were in the non-PH cohort. The mean age of PH patients was 48.1 years, and approximately 58% of the sample were male. Significantly more patients in the PH cohort than the non-PH cohort were diagnosed with stage 2 chronic kidney disease (CKD; 3.1% vs 0.4%, respectively; P < 0.001), stage 3 CKD (4.6% vs 0.5%; P < 0.001), stage 4 CKD (2.5% vs 0.1%; P < 0.001), and stage 5 CKD or end-stage renal disease (ESRD; 2.2% vs 0.1%; P < 0.001). PH patients had a significantly higher mean Charlson Comorbidity Index composite score than patients in the non-PH cohort (0.79 vs 0.37; P < 0.001). HCRU was significantly higher in patients with PH. The PH cohort had a significantly higher proportion of patients with at least 1 visit to clinicians specializing in nephrology (19% vs 0.4%, respectively; P < 0.001), cardiology (22% vs 12%; P < 0.001), ophthalmology (16% vs 7%; P < 0.001), general surgery (9% vs 6%; P = 0.011), and urology (65% vs 6%; P < 0.001) compared with patients without PH. Mean total annual health care costs in the PH cohort were 65% higher than in the non-PH cohort ($22,549 vs $7,852, respectively; P < 0.001). Similar results were found for total prescription drug costs ($4,125 vs $2,464; P = 0.012).
CONCLUSIONS:
Despite the rarity of PH, patients with this disease incur substantial clinical and economic burden and may cause financial strain on the health care system. Additional research is warranted to understand the economic and clinical burden of PH stratified by the 3 subtypes of the disease.
What is already known about this subject
Primary hyperoxaluria (PH) is a debilitating disease, yet very little is known about the health care utilization and costs associated with specific types of care among PH patients.
Characterization of the burden of illness of PH was challenging before 2018 because there was no ICD-10 code for PH, but with the advent of this code, researchers have been able to discern costs and utilization specific to PH patients.
What this study adds
This study quantifies health care utilization and costs for patients with PH.
Findings demonstrate that health care costs for PH patients are often significantly higher than those in a matched cohort without the disease.
Primary hyperoxaluria (PH) is a family of rare, life-threatening genetic liver disorders characterized by elevated production and excretion of oxalate.1,2 Three subtypes of PH have been identified, each caused by a genetic mutation and resulting in a distinct enzyme deficiency; all PH subtypes ultimately lead to the overproduction of oxalate in the liver, as well as an increase in the excretion of oxalate by the kidneys.1,2 Excess oxalate combines with calcium in the kidneys to form calcium oxalate, the primary component of kidney stones; consequently, patients with PH often experience recurrent kidney stones starting at a young age, with the mean age of PH symptom onset around 7.5 years (median: 4.1 years).3,4
An additional outcome of excessive oxalate can be the development of calculi throughout the urinary tract (urolithiasis) and kidneys (nephrolithiasis), obstruction of the renal tubules,5 and a progressive increase of calcium-oxalate deposition in the kidneys (nephrocalcinosis).6 A chronic inflammatory process induced by the internalization of calcium oxalate crystals in the kidneys may lead to chronic kidney disease (CKD) or end-stage renal disease (ESRD) in tandem with systemic oxalosis, a process by which calcium oxalate crystals are deposited throughout various organ systems.2,5 Systemic oxalosis often affects bone, skin, retina, myocardium, blood vessels, and even the central nervous system, potentially leading to serious complications or death if left untreated.2
The prevalence of PH is estimated to be less than 3 diagnosed cases per 1,000,000 individuals.1 In contrast, whole-genome sequencing studies have suggested that the prevalence of PH (based on known mutations) is approximately 1 case in 58,000 individuals, suggesting that many cases are undiagnosed or misdiagnosed; thus, the prevalence of PH may be greatly underestimated due to some patients presenting with relatively mild symptoms or becoming symptomatic only later in life.1
The management of PH varies depending on the type, severity, and progression of the disease,2 with only 1 pharmacologic treatment (lumasiran) approved by the US Food and Drug Administration for the treatment of PH type 1.7 Approaches to treatment for patients in early stages of PH also may consist of increasing fluid intake and pyridoxine (vitamin B6 in PH1) to help dilute and reduce endogenous oxalate production (B6) and thus urinary oxalate.5,8 Administration of potassium or sodium citrate may also be beneficial for urine alkalinization and an increase of urinary citrate excretion, thereby inhibiting calcium oxalate crystallization.8 PH patients may also progress to ESRD requiring dialysis, making isolated kidney transplantation or combined liver-kidney transplantation, in selected cases, necessary.2,6
Because of the rarity of PH and, until recently, lack of diagnostic coding to enable identification of this specific diagnosis through claims data analyses, the clinical and economic burden of PH has not been well documented. However, patients with PH can now be identified by International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes so assessing the burden directly associated with PH is now possible. Thus, the objective of the present study was to characterize the clinical and economic costs and health care resource utilization (HCRU) associated with diagnosed PH relative to a matched cohort of patients without the disease or any official diagnosis of PH.
Methods
STUDY DESIGN AND DATA SOURCE
A retrospective, matched cohort study using commercial claims data from the IQVIA PharMetrics Plus Database was performed. The PharMetrics Plus Database contains demographic information, health plan enrollment, medical and pharmacy claims, characteristics of HCRU (including place of service), and ICD-10-CM codes for over 140 million Americans with health insurance. Differences in clinical characteristics, HCRU, and pharmacy and medical costs were evaluated in patients with PH compared with a matched cohort of patients without PH from January 2014 to December 2019.
STUDY POPULATION SELECTION
Two cohorts were constructed for this analysis. The first was the PH cohort, which included patients of any age who had at least 1 claim reported on or after October 2018 with a PH diagnosis (ICD-10-CM code E72.53) and were continuously enrolled in both medical and prescription benefits for at least 1 year before and following the diagnostic code. Before October 2018, the hyperoxaluria code included primary and secondary types, so the ICD code was only used after October 2018, when it became specific to PH.
The second cohort consisted of non-PH patients. For this cohort, a 5% random sample of patients of any age who did not have any of the diagnoses (including PH or calculi [stones]) was drawn from the dataset (Supplementary Table 1 (26.7KB, pdf) , available in online article). However, in order to mitigate bias by the exclusion of calculi in the non-PH cohort, an additional 5% random sample of patients with calculi but no PH diagnosis was drawn and then restored to the non-PH cohort. Patients who were not continuously enrolled in medical and prescription benefits for the study period were excluded. The remaining patients were then randomly matched to patients with PH (5 patients without PH to 1 patient with PH) based on age, sex, and insurance type.9 Patients in the non-PH cohort who did not match to a patient with PH were excluded from the analyses.
PATIENT DEMOGRAPHICS AND CLINICAL OUTCOMES
Patient demographics such as age, sex, race, region, and insurance type were recorded and compared between patients in the PH and non-PH cohorts. Clinical outcomes of interest included occurrence of kidney stones and urinary tract infections, CKD stages, acute kidney failure, kidney or liver transplantation, and dialysis.
Comorbidities of patients in the PH and non-PH cohorts were compared using the Quan et al adapted version of the Charlson Comorbidity Index (CCI).10 The index consists of 12 diseases weighted to sum to a score ranging from 0-24, with a higher score indicating a greater disease burden. For the present analyses, the percentage of patients with a diagnosis for each comorbidity was calculated in addition to the mean CCI scores for patients in the PH and non-PH cohorts.
HEALTH CARE RESOURCE UTILIZATION
HCRU was defined as the total number of inpatient, outpatient, emergency department, and physician office visits, as well as prescription drug claims for a 12-month period after the index date. The annual number of patient visits to select physician specialties, including nephrology, cardiology, ophthalmology, pediatrics, general surgery, and urology, were also reported based on the number of patients with at least 1 encounter in any of those specialty areas.
HEALTH CARE COSTS
Total medical and prescription drug costs were adjusted to the 2019 medical care component of the Consumer Price Index and reflected the 12-month costs across all patients in each cohort.
Mean prescription costs were compared for patients in the PH and non-PH cohorts by examining the 15 costliest prescription drug classes used by patients in the PH cohort. In addition to these 15 medication classes, the mean and median costs of opioid and narcotic analgesics (as a drug class) were examined. Although opioid use has yet to be investigated in patients with PH, use of opioid or narcotic analgesic drugs (as a class) was of interest, since individuals with PH reportedly deal with high levels of pain.11
STATISTICAL ANALYSIS
All analyses were performed using SAS/STAT 14.3 (SAS Institute Inc.) and R 3.6.2 (R Foundation). Categorical variables were reported as percentages, while continuous variables were reported as means and SDs. The median value is only shown for total health care costs. Significant differences between cohorts were examined using the bootstrap t-test and Wilcoxon rank sum test. For categorical variables, the chi square test was used to test for statistically significant differences in proportions.
Results
PATIENT DEMOGRAPHICS AND CLINICAL OUTCOMES
A total of 1,944 patient records (hereafter referred to as “patients”) were included in the study (PH cohort, n = 324; non-PH cohort, n = 1,620; Figure 1). Most PH patients (96%) had commercial insurance, and 4% had Medicaid or Medicare (Table 1). The mean (SD) age of patients was 48.1 (15.8) years, and approximately 58% of the sample was male.
FIGURE 1.
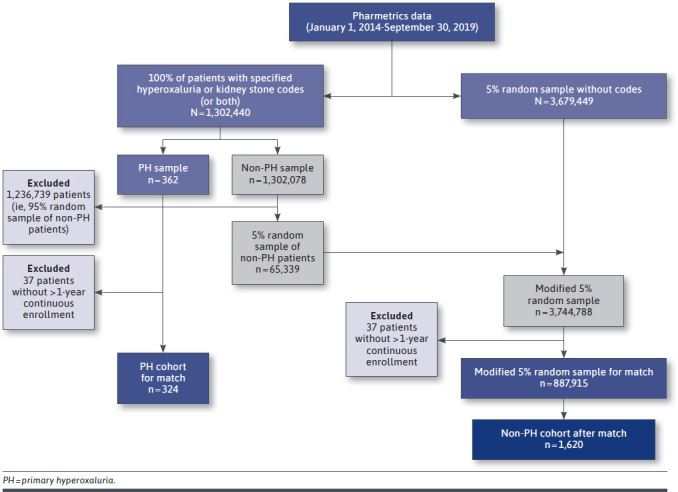
Sample Construction Flow Chart
TABLE 1.
Patient Demographic Characteristics
| PH n = 324 | % | Non-PH n = 1,620 | % | |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 48.1 (15.8) | – | 48.1 (15.8) | – |
| Age distribution, years | ||||
| < 18 | 20 | 6 | 100 | 6 |
| 18-35 | 41 | 13 | 205 | 13 |
| 36-55 | 137 | 42 | 685 | 42 |
| 55+ | 126 | 39 | 630 | 39 |
| Sex | ||||
| Male | 188 | 58 | 940 | 58 |
| Female | 136 | 42 | 680 | 42 |
| Region | ||||
| Northeast | 57 | 18 | 257 | 16 |
| Midwest | 104 | 32 | 477 | 29 |
| South | 125 | 39 | 692 | 43 |
| West | 38 | 12 | 194 | 12 |
| Insurance | ||||
| Commercial | 312 | 96 | 1,560 | 96 |
| Medicaid | 9 | 3 | 45 | 3 |
| Medicare | 3 | 1 | 15 | 1 |
PH = primary hyperoxaluria.
Significantly more patients in the PH cohort than the non-PH cohort were diagnosed with stage 2 CKD (3.1% vs 0.4%, respectively; P < 0.001), stage 3 CKD (4.6% vs 0.5%; P < 0.001), stage 4 CKD (2.5% vs 0.1%; P < 0.001), and stage 5 CKD or ESRD (2.2% vs 0.1%; P < 0.001; Table 2). A greater proportion of patients in the PH cohort than the non-PH cohort had a history of kidney stones before study entry (35% vs 0.9%, respectively; P < 0.001). Among those patients who had a stone in the 12-month study period, a greater proportion of patients in the PH cohort than in the non-PH cohort had 2 or more stones (59% vs 24%, respectively; P < 0.001). Despite the sample being more heavily representative of patients in lower CKD stages, a small proportion of PH patients underwent organ transplantation during the study (2.2% kidney, 1.2% liver); however, no patients in the non-PH cohort underwent kidney or liver transplantation during the study period.
TABLE 2.
Patient Clinical Characteristics
| PH, % n = 324 | Non-PH, % n = 1,620 | P value | |
|---|---|---|---|
| History of kidney stones | 35 | 0.9 | < 0.001 |
| ≥ 1 kidney stone occurrence in the study period | 80 | 3 | < 0.001 |
| ≥ 2 occurrencesa | 59 | 24 | < 0.001 |
| CKD, by stage | |||
| Stage 1 | 0.6 | 0.2 | 0.273 |
| Stage 2 | 3.1 | 0.4 | < 0.001 |
| Stage 3 | 4.6 | 0.5 | < 0.001 |
| Stage 4 | 2.5 | 0.1 | < 0.001 |
| Stage 5 or ESRD | 2.2 | 0.1 | < 0.001 |
| Acute kidney failure | 6.5 | 1.1 | < 0.001 |
| Kidney transplantation | 2.2 | 0.0 | < 0.001 |
| Liver transplantation | 1.2 | 0.0 | < 0.001 |
| ≥ 1 UTI | 18 | 3.1 | < 0.001 |
| ≥ 2UTIs among patients with ≥ 1 UTI | 29 | 10 | 0.013 |
a Among patients with ≥ 1 kidney stone.
CKD = chronic kidney disease; ESRD = end-stage renal disease; PH = primary hyperoxaluria; UTI = urinary tract infection.
A significantly greater proportion of patients in the PH cohort relative to the non-PH cohort were diagnosed with 1 or more of the following comorbidities via presence of the disease-specific ICD-10-CM code on a claim (all P < 0.001): peripheral vascular disease (5.2% vs 2.0%), cerebrovascular disease (4.3% vs 1.4%), mild liver disease (9.9% vs 3.5%), diabetes without chronic complications (18% vs 10%), hemiplegia or paraplegia (2.2% vs 0.2%), and moderate or severe renal disease (10% vs 1.3%; Supplementary Table 2 (26.7KB, pdf) , available in online article). Patients in the PH cohort had a significantly higher mean CCI composite score, indicating greater disease burden, than patients in the non-PH cohort (0.79 vs 0.37, respectively; P < 0.001).
HEALTH CARE RESOURCE UTILIZATION
HCRU was significantly higher in the PH cohort across most types of care in the aggregate included in this study. When prespecified specialty care was examined individually, the PH cohort had a significantly higher proportion of patients with at least 1 visit to clinicians specializing in nephrology (19% vs 0.4%, respectively; P < 0.001), cardiology (22% vs 12%; P < 0.001), ophthalmology (16% vs 7%; P < 0.001), general surgery (9% vs 6%; P = 0.011), and urology (65% vs 6%; P < 0.001; Table 3). The lone exception was visits to a pediatrician; no significant between-cohort difference was observed (P = 0.139), which may have been attributable to an insufficient pediatric sample size in this analysis.
TABLE 3.
Health Care Resource Utilization and Costs
| PHn = 324Mean (SD) | Non-PHn = 1,620Mean (SD) | P value | |
|---|---|---|---|
| Health care resource utilization | |||
| Outpatient visits | 23 (21) | 9 (13) | < 0.001 |
| Inpatient visits | 0.8 (4.2) | 0.2 (2.2) | < 0.001 |
| Emergency department visits | 0.7 (1.4) | 0.2 (0.6) | < 0.001 |
| Physician office visits | 13 (14) | 6 (8) | < 0.001 |
| Patients with ≥ 1 visit to any specialist, % | |||
| Urologist | 65 | 6 | < 0.001 |
| Cardiologist | 22 | 12 | < 0.001 |
| Nephrologist | 19 | 0.4 | < 0.001 |
| Ophthalmologist | 16 | 7 | < 0.001 |
| General surgeon | 9 | 6 | 0.011 |
| Pediatrician | 7 | 5 | 0.139 |
| Health care costs, $ | |||
| Total mean costs (SD) | 22,549 (36,716) | 7,852 (23,552) | < 0.001 |
| Total median costs | 11,017 | 1,685 | < 0.001 |
| Mean costs by type of care | |||
| Outpatient visits | 13,894 (19,444) | 3,784 (8,636) | < 0.001 |
| Inpatient visits | 4,530 (18,176) | 1,605 (12,282) | < 0.001 |
| Prescription drugs | 4,125 (10,620) | 2,464 (13,311) | < 0.001 |
| Outpatient surgery | 3,379 (6,514) | 785 (3,180) | < 0.001 |
| Patient cost-share amount | 2,737 (2,728) | 1,145 (1,927) | < 0.001 |
| Physician office visits | 1,759 (2,126) | 684 (1,014) | < 0.001 |
| Emergency department visits | 651 (1,250) | 211 (769) | < 0.001 |
PH = primary hyperoxaluria.
HEALTH CARE COSTS
Regardless of setting, all mean annual health care costs were significantly higher for patients in the PH cohort than in the non-PH cohort (Table 3). For total health care costs, mean and median costs were significantly higher in the PH cohort; mean costs were 2.9 times higher in the PH cohort than in the non-PH cohort ($22,529 vs $7,852, respectively; P < 0.001), and median costs were 6.5 times higher ($11,017 vs $1,685; P < 0.001). The differentials for individual types of care continued to exhibit a pattern of higher utilization in the PH cohort. The highest mean costs in the 12-month study period for the PH cohort compared with the non-PH cohort were in outpatient visits ($13,894 vs $3,784, respectively; P < 0.001) followed by inpatient visits ($4,530 vs $1,605; P < 0.001). The percentage difference in costs for care was consistently higher in the PH cohort and ranged from 40% higher for prescription drugs to 77% higher for outpatient surgery.
Prescription drug annual costs were reported in the aggregate and for selected individual classes. The mean total cost of prescription drugs was 1.7 times higher for patients in the PH cohort than for patients in the non-PH cohort ($4,125 vs $2,464, respectively; P < 0.001). Among the top 15 most expensive prescription drug categories used by patients in the PH cohort, several significant between-cohort differences were observed (Figure 2).
FIGURE 2.
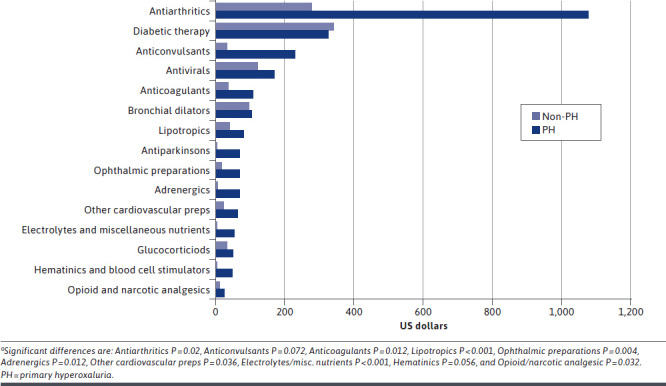
Prescription Drug Costsa by Class
The mean costs of prescriptions were significantly higher for patients in the PH cohort compared with the non-PH cohort for antiarthritics ($1,077 vs $278, respectively; P = 0.020), anticoagulants ($107 vs $35; P = 0.012), lipotropics ($80 vs $38; P < 0.001), adrenergics ($69 vs $4; P = 0.012), ophthalmic preparations ($69 vs $18; P = 0.004), and electrolytes/miscellaneous nutrients ($56 vs $2; P < 0.001).
While not included in the top 15 most expensive drug classes, a significantly greater proportion of patients in the PH cohort were prescribed opioids or narcotic analgesics compared with patients in the non-PH cohort (47% vs 15%, respectively; P < 0.001).
Discussion
To our knowledge, this is the first study to describe HCRU and the overall economic burden of patients with PH compared with those with no diagnostic claim for PH. Overall, our results indicate that patients with PH generally used more health care resources and generated greater health care and prescription drug annual costs relative to their matched non-PH counterparts. As expected, a greater proportion of patients with PH experienced 1 or more kidney stone events and had a diagnosis of stages 2-5 CKD. As expected, a greater number of patients in the PH cohort had renal or liver disease than in the non-PH cohort. What was unexpected, however, was that the comorbidity most frequently identified as part of the components of the CCI scores for patients with PH was diabetes without chronic complications (CCI does not stratify by type I or II; 18% of patients with PH vs 10% of non PH patients; P < 0.001). Further research is required to determine if there is a relationship, causal or otherwise, between diabetes and PH.
Our findings also suggest that a greater proportion of patients with PH consulted specialists in nephrology, cardiology, ophthalmology, and urology compared with those without PH. While this may be a function of needed treatment for comorbidities and the sequelae of PH itself, these results were consistent with other outcomes in this study, indicating that patients with PH use more health care resources and generate greater overall costs than their matched non-PH controls. While higher rates of utilization among patients in the PH cohort were expected for specialty areas related to PH (eg, urology), the elevated utilization in other areas, such as cardiology and ophthalmology, were unexpected. Additional research will be needed to explore this further.
Results of this study demonstrated the substantial economic costs associated with PH. Total health care annual costs were substantially higher in the PH cohort, indicating that the burden of PH is pervasive across different health care settings. Total prescription drug costs were also higher for PH patients compared with non-PH patients, despite many of the classes, including generic agents, and no approved therapeutics for PH being available during the data collection period. The impact of available (such as lumasiran) or upcoming pharmacologic treatments for PH on prescription drug costs should be the subject of additional research. Additionally, a greater proportion of patients in the PH cohort had claims for opioid and narcotic analgesics than in the non-PH cohort. While this may be related to pain management of renal sequelae, such as kidney stones, additional research is needed to establish a causal link.
LIMITATIONS
As with all claims database analyses, there are inherent limitations to the present study. ICD codes before October 2018 did not distinguish between primary and secondary hyperoxaluria. For this reason, our analyses were limited to patients with the PH specific ICD code appearing after October 2018. It is also assumed that the coding was accurate, although the possibility of inaccurate and inconsistent coding exists. Furthermore, the ICD-10 code did not allow stratification by PH subtypes 1, 2, and 3.
Our sample contained a very low proportion of pediatric patients (aged < 18 years) and renally compromised patients; therefore, the true clinical and economic burden of PH may have been underestimated. It is not clear why claims for this rare condition were not seen in a greater number of pediatric patients in this claims database. Also, while the likelihood of including undiagnosed PH patients in the non-PH group was low, it may have happened. Given the lack of a validated algorithm to accurately identify undiagnosed patients, it was not possible to detect them via claims and exclude them from the non-PH group. If this did occur, it may have been so few patients that fell into the initial 3.7 million claims reviewed for the non-PH group that their influence on the results would be negligible at best.
Results of the current study were based primarily on commercial claims data, so our findings may not be generalizable to patients with noncommercial payer types (eg, Medicare and Medicaid). The small proportion of Medicare patients also would have been an indicator of underrepresentation of patients with ESRD. Inclusion in this study required continuous enrollment, which may have excluded patients who switched insurance providers.
Finally, this study did not link prescription drugs to the patients’ ICD-10-CM codes, so the indication that the prescriptions were written for was not specified.
Conclusions
The results presented here suggest that PH leads to a substantial clinical and economic burden for patients and health care systems. Although PH is a rare disease, patients were found to incur substantial costs across most types of health care. This demonstrated that a small number of diagnosed PH patients can still incur significant costs and clinical outcomes, such as chronic comorbidities and sequelae commonly found in PH (eg, kidney stones). Further research is needed to evaluate the burden of PH stratified by subtype and to assess the impact of advanced disease stage on clinical and economic outcomes.
REFERENCES
- 1.Hopp K, Cogal AG, Bergstralh EJ, et al. Phenotype-genotype correlations and estimated carrier frequencies of primary hyperoxaluria. J Am Soc Nephrol. 2015;26(10):2559-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369(7):649-68. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin B, Ürekli HM, Atta MG. Primary and secondary hyperoxaluria: understanding the enigma. World J Nephrol. 2015;4(2):235-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sas DJ, Enders FT, Mehta RA, et al. Clinical features of genetically-confirmed patients with primary hyperoxaluria identified by clinical indivation versus familial screening. Kidney Int. 2021;97(4):786-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Shalom E, Frishberg Y. Primary hyperoxalurias: diagnosis and treatment. Pediatr Nephrol. 2015;30(10):1781-91. [DOI] [PubMed] [Google Scholar]
- 6.Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8(8):467-75. [DOI] [PubMed] [Google Scholar]
- 7.Oxlumo. Prescription information. Alnylam Pharmaceuticals, Inc; 2020. Accessed January 8, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214103lbl.pdf [Google Scholar]
- 8.Harambat J, Fargue S, Bacchetta J, Acquaviva C, Cochat P. Primary hyperoxaluria. Int J Nephrol. 2011;2011: 864580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoffstall AJ, Gaebler JA, Kreher NC, et al. The high direct medical costs of Prader-Willi syndrome. J Pediatr. 2016;175:137-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-82. [DOI] [PubMed] [Google Scholar]
- 11.Cochat P, Groothoff J. Primary hyperoxaluria type 1: practical and ethical issues. Pediatr Nephrol. 2013;28(12):2273-81. [DOI] [PubMed] [Google Scholar]


