Abstract
Background
Older adults hospitalized for heart failure (HF) are at risk for falls after discharge. One modifiable contributor to falls is fall risk-increasing drugs (FRIDs). However, the prevalence of FRIDs among older adults hospitalized for HF is unknown. We describe patterns of FRIDs use and examine predictors of a high FRID burden.
Methods
We used the national biracial REasons for Geographic and Racial Differences in Stroke (REGARDS) study, a prospective cohort recruited from 2003–2007. We included REGARDS participants aged ≥ 65 years discharged alive after a HF hospitalization from 2003–2017. We determined FRIDs –cardiovascular (CV) and non-cardiovascular (non-CV) medications – at admission and discharge from chart abstraction of HF hospitalizations. We examined the predictors of a high FRID burden at discharge via modified Poisson regression with robust standard errors.
Results
Among 1147 participants (46.5% women, mean age 77.6 years) hospitalized at 676 hospitals, 94% were taking at least 1 FRID at admission and 99% were prescribed at least 1 FRID at discharge. The prevalence of CV FRIDs was 92% at admission and 98% at discharge, and the prevalence of non-CV FRIDs was 32% at admission and discharge. The most common CV FRID at admission (88%) and discharge (93%) were antihypertensives; the most common agents were beta blockers (61% at admission, 75% at discharge), angiotensin-converting enzyme inhibitors (36% vs. 42%), and calcium channel blockers (32% vs. 28%). Loop diuretics had the greatest change in prevalence (53% vs. 72%). More than half of the cohort (54%) had a high FRID burden (Agency for Healthcare Research and Quality (AHRQ) score ≥ 6), indicating high falls risk after discharge. In a multivariable Poisson regression analysis, the factors strongly associated with a high FRID burden at discharge included hypertension (PR: 1.41, 95% CI: 1.20, 1.65), mood disorder (PR: 1.24, 95% CI: 1.10, 1.38), and hyperpolypharmacy (PR: 1.88, 95% CI: 1.64, 2.14).
Conclusions
FRID use was nearly universal among older adults hospitalized for HF; more than half had a high FRID burden at discharge. Further work is needed to guide the management of a common clinical conundrum whereby guideline indications for treating HF may contribute to an increased risk for falls.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-023-03401-w.
Keywords: Falls, Heart failure, Fall risk-increasing drugs
Introduction
Falls are a concern among older adults given their impacton morbidity and mortality [1]. Almost one-third of older adults who fall suffer physical harm [2, 3]. Resulting hip fractures are especially relevant as they lead to decreased life expectancy, diminished functional capacity, and loss of independence [4, 5]. Older adults with HF are predisposed to a high risk for falls for multiple reasons. These include age-related physiologic alterations, such as decreased autonomic reflexes, reduced adrenergic responsiveness, and intravascular volume contraction, which contribute to orthostatic hypotension [6]; arrhythmias which can lead to impaired cardiac output and cardiac syncope [7]; and geriatric conditions such as cognitive impairment and frailty, predispose older adults to falls [8, 9]. Moreover, being in the hospital is a major risk factor for falls [10], and can be exacerbated by delirium [11]. Finally, polypharmacy, which is nearly universal in older adults hospitalized for HF, is also a major risk factor for falls [12–14]. Despite these risk factors, older adults are often prescribed HF-specific medications in addition to medications for other conditions which increase the risk for hypotension, dizziness, and subsequent falls [12, 15, 16].
Consequently, clinicians often face a conundrum when caring for older adults hospitalized for HF—medications to treat HF and other conditions can improve long-term outcomes, but might do so at an increased risk of falls. An improved understanding of the potentially modifiable contributors to fall risk in older adults hospitalized for HF could inform future intervention development to address this conundrum. Indeed, a modifiable contributor to falls could be the use of fall risk-increasing drugs (FRIDs), a group of medications with the potential to increase the risk of falls through effects on the cardiovascular (CV) and central nervous systems [17–19]. To our knowledge, the prevalence of FRIDs among older adults hospitalized for HF has not been described. To address this knowledge gap and create a foundation for future work to reduce risk of falls in this population, we characterized the prescribing patterns of FRIDs at the time of admission and discharge for a HF hospitalization, and examined the determinants of FRIDs prescribing at hospital discharge.
Methods
Study population
The REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study is a prospective cohort study that includes 30,239 White and Black community-dwelling men and women aged ≥ 45 years at enrollment recruited between 2003–2007 from all 48 contiguous states of the United States with ongoing follow-up [20]. The REGARDS study was originally designed to determine antecedents of racial and geographic differences in stroke mortality in the United States, and has since been used to study CV diseases including HF [12, 15, 21, 22]. Participants were randomly sampled, with oversampling in the Southeastern US and of Black participants. Participant information such as demographics and health behaviors were elicited at baseline through computer assisted telephone interviews and an in-home visit. CV outcomes and related hospitalizations are detected every 6 months via telephone and retrieval of medical records. Two expert clinician adjudicators determine whether hospitalizations are caused by HF based on review of medical records from the hospitalization; a third adjudicator is employed to resolve any disagreements. The institutional review boards of all collaborating institutions approved the REGARDS study protocol and all participants provided written informed consent at the time of enrollment.
For this study, we studied participants aged ≥ 65 years who were discharged alive after experiencing an adjudicated HF hospitalization between 2003 and 2017. We excluded participants referred to hospice at hospital discharge and excluded participants who did not have medication data at both hospital admission and discharge. In the case of multiple hospitalizations for a participant, we examined the first hospitalization.
Data sources
We utilized data from three merged datasets: 1) the REGARDS study baseline assessment and periodic cognitive assessments; 2) medical record chart abstraction from adjudicated HF hospitalizations within REGARDS; 3) the American Hospital Association (AHA) Annual Survey Database, which contains information on > 6,500 hospitals across the US.
Baseline characteristics included age, sex, self-identified race, income, education, functional impairment (Physical Component Summary score < 40 from the Short Form 12-item questionnaire [23]. Patients were also asked about a history of falls, defined as at least one self-reported fall in the prior year. The 6-item screener, a short assessment of cognitive functioning, was administered during follow-up calls every 2 years; the cognitive assessment completed most closely and prior to the hospitalization was used, with cognitive impairment defined as a 6-item screener score < 5 [24].
Chart abstraction included data from admission notes, progress notes, discharge summaries, and medication reconciliation reports. We abstracted patients’ medical conditions, admission and discharge vital signs, laboratory values, echocardiogram parameters such as left ventricular ejection fraction (LVEF), medications prescribed on admission and discharge, and hospitalization factors such as length of stay, intensive care unit (ICU) stay, and discharge disposition. HF with preserved ejection fraction (HFpEF) was defined as LVEF ≥ 50% or a qualitative description of normal systolic function [25], and HF with a reduced ejection fraction (HFrEF) was defined as LVEF < 50% or a qualitative description of abnormal systolic function [26]. We grouped patients with HF with a mildly reduced ejection fraction (HFmrEF) with those with HFrEF given shared pathophysiology and response to treatment [26].
AHA Annual Survey data from 2003–2017 included hospital size, where a small hospital size was defined as < 200 beds; and academic/teaching status, defined as inclusion in the Association of American Medical Colleges Council of Teaching Hospitals and Health Systems or certification by the Accreditation Council for Graduate Medical Education.
Fall risk-increasing medications (FRIDs)
We identified fall risk-increasing medications (FRIDs) as medications associated with increased fall risk based on comprehensive systematic reviews and meta-analyses [18, 19, 27–29]. We classified FRIDs into two main categorizations: CV FRIDs and non-CV FRIDs. CV FRIDs included antihypertensives (some of which are classically described as guideline-directed medical therapy for HF), including beta-blockers, renin-angiotensin system inhibitors, calcium channel blockers, nitrates, alpha blockers, vasodilators, alpha agonists, digoxin, and diuretics (thiazides, loop diuretics, potassium-sparing diuretics). Non-CV FRIDs included antidepressants, benzodiazepines, opioids, antiepileptics, and antipsychotics. Classification of CV vs. non-CV was based on a classification scheme previously used and primarily based on the Multum Lexicon Drug Database [12, 30].
To quantify FRID burden at hospital discharge, we used the Agency for Healthcare Research and Quality (AHRQ) medication-based fall risk tool [31]. This tool provides a weighted risk score for falls based on the medication regimen. To calculate the AHRQ score, each medication is assigned a score (higher scores indicate higher risk for falls), and then scores are then summed to generate a weighted score. Medication scores were calculated as follows: antihypertensives = 2, digoxin = 2, loop diuretics = 1. For non-CV FRIDs, drugs were assigned a score of 3, with the exception of antidepressants which were assigned a score of 2.
Statistical analysis
To summarize participant and hospital characteristics, we calculated medians and interquartile ranges (IQR) for continuous variables and percentages for categorical variables. We also calculated the prevalence and median counts for all FRIDs, CV FRIDs, and non-CV FRIDs at admission and at discharge; and calculated the change between admission and discharge.
We calculated the prevalence of participants with a high FRID burden, and specified a multivariable modified Poisson regression model with robust standard errors to estimate prevalence ratios with 95% confidence intervals (CIs) to identify predictors of a high FRID burden at hospital discharge. High FRID burden was defined by an AHRQ medication-based fall risk score of ≥ 6, which is a threshold indicating excess risk [31, 32]. Candidate covariates in the model included: socio-demographics (age, sex, race, income, education), HF subtype (HFrEF vs. HFpEF), comorbid conditions for which FRIDs may be prescribed (hypertension, atrial fibrillation/atrial flutter, mood disorder, osteoarthritis, rheumatoid arthritis, and cancer), discharge medications that may impact FRIDs prescribing (anticoagulants), geriatric conditions (functional impairment, cognitive impairment, history of falls in the year prior to REGARDS study enrollment, hyperpolypharmacy – defined as the use of at least 10 medications at discharge), hospitalization factors (length of stay, intensive care unit (ICU) stay, geriatric/palliative involvement, discharge disposition), and hospital characteristics (teaching status, hospital size). A p-value < 0.05 was considered significant. To account for missing covariate values, we used multiple imputation via chained equations [33]. Of note, the covariates with the highest degree of missingness were HF subtype (35%), cognitive impairment (27%), and income (13%). We managed the data in SAS version 9.4 (SAS Institute, Cary, NC) and performed statistical analysis using STATA version 17 (StataCorp LLC, College Station, TX).
Results
We examined 1147 unique participants hospitalized for HF. The median age was 78 years (IQR: 72,83 years), 47% were women, and 40% were Black persons (Table 1). The most common comorbid condition was hypertension (79%). The median number of medications taken at hospital admission was 9 (IQR: 6, 12) and at discharge was 10 (IQR: 8, 13). The prevalence of hyperpolypharmacy was 45% at admission and 57% at discharge.
Table 1.
Baseline characteristics according to FRIDs use at admission
| Characteristics | All | FRIDS | |
|---|---|---|---|
| Absent | Present | ||
| N | 1147 | 67 | 1080 |
| Age, years | 77.6 (72.2, 83.1) | 76.8 (73.8, 82.4) | 77.73 (72.14, 83.23) |
| Black | 456 (39.8%) | 18 (26.9%) | 438 (40.6%) |
| Female | 533 (46.5%) | 26 (38.8%) | 507 (46.9%) |
| Education less than high school | 243 (21.2%) | 16 (23.9%) | 227 (21.0%) |
| Income < $20,000 | 286 (28.5%) | 15 (25.0%) | 271 (28.7%) |
| Clinical Characteristics | |||
| HFrEF | 395 (52.8%) | 20 (48.8%) | 375 (53.0%) |
| Hypertension | 899 (78.5%) | 26 (39.4%) | 873 (80.9%) |
| Atrial fibrillation/atrial flutter | 482 (42.0%) | 29 (43.3%) | 453 (41.9%) |
| Mood disorder | 176 (15.3%) | 3 (4.5%) | 173 (16.0%) |
| Osteoarthritis | 298 (26.0%) | 11 (16.4%) | 287 (26.6%) |
| Rheumatoid arthritis | 27 (2.4%) | 5 (7.5%) | 22 (2.0%) |
| Cancer | 219 (19.1%) | 9 (13.4%) | 210 (19.4%) |
| Anticoagulant use | 352 (30.7%) | 24 (35.8%) | 328 (30.4%) |
| Hyperpolypharmacy (at discharge) | 657 (57.3%) | 20 (29.9%) | 637 (59.0%) |
| Functional impairment | 461 (43.0%) | 13 (21.0%) | 448 (44.3%) |
| Cognitive impairment | 125 (14.9%) | 6 (12.8%) | 119 (15.0%) |
| History of falls (in the past year) | 233 (20.4%) | 9 (13.4%) | 224 (20.8%) |
| Hospitalization Factors | |||
| Length of stay (days) | 5.0 (3.0, 8.0) | 5.5 (4.0, 9.0) | 5.00 (3.00, 8.00) |
| ICU stay during hospitalization | 211 (18.4%) | 20 (30.3%) | 191 (17.7%) |
| Geriatric/palliative involvement | 13 (1.1%) | 1 (1.5%) | 12 (1.1%) |
| Disposition | |||
| Home | 908 (79.2%) | 52 (77.6%) | 856 (79.3%) |
| Institution | 185 (16.1%) | 9 (13.4%) | 176 (16.3%) |
| Alive, unknown disposition | 54 (4.7%) | 6 (9.0%) | 48 (4.4%) |
| Hospital Characteristics | |||
| Teaching status | 527 (51.5%) | 31 (50.0%) | 496 (51.6%) |
| Small hospital size (< 200 beds) | 262 (25.1%) | 13 (21.0%) | 249 (25.4%) |
Values are n (%), unless otherwise indicated
Abbreviations: HF Heart failure, HFrEF Heart failure with reduced ejection fraction, ICU Intensive care unit
The prevalence of FRIDs use at hospital admission was 94% and at discharge was 99% (Tables 1 and 2, Supplemental Tables 1-2). The median number of FRIDs at both hospital admission and discharge was 3 (IQR: 2, 4). At admission, 37% took at least 4 FRIDS; and at discharge, 47% took at least 4 FRIDs (Fig. 1). Nearly half (42%) of patients were taking at least one additional FRID at discharge compared to admission. The most commonly initiated FRIDs (present on discharge, absent on admission) were loop diuretics (26%) and beta blockers (18%). The two most commonly discontinued FRIDs (present on admission, absent on discharge) were thiazides (10%) and calcium channel blockers (CCB) (10%). These patterns were similar among those with HFrEF and HFpEF (Supplemental Fig. 1-2 and Supplemental Table 3).
Table 2.
Baseline characteristics according to FRIDs use at discharge
| Characteristics | FRIDs | |
|---|---|---|
| Absent | Present | |
| N | 15 | 1132 |
| Age, years | 76.6 (70.3, 83.0) | 77.7 (72.3, 83.2) |
| Black | 5 (33.3%) | 451 (39.8%) |
| Female | 9 (60.0%) | 524 (46.3%) |
| Education less than high school | 3 (20.0%) | 240 (21.2%) |
| Income < $20,000 | 5 (41.7%) | 281 (28.4%) |
| Clinical Characteristics | ||
| HFrEF | 2 (16.7%) | 393 (53.4%) |
| Hypertension | 6 (40.0%) | 893 (79.0%) |
| Atrial fibrillation/atrial flutter | 7 (46.7%) | 475 (42.0%) |
| Mood disorder | 4 (26.7%) | 172 (15.2%) |
| Osteoarthritis | 4 (26.7%) | 294 (26.0%) |
| Rheumatoid arthritis | 1 (6.7%) | 26 (2.3%) |
| Cancer | 3 (20.0%) | 216 (19.1%) |
| Anticoagulant use | 5 (33.3%) | 347 (30.7%) |
| Hyperpolypharmacy (at discharge) | 3 (20.0%) | 654 (57.8%) |
| Functional impairment | 6 (42.9%) | 455 (43.0%) |
| Cognitive impairment | 2 (18.2%) | 123 (14.8%) |
| History of falls (in the past year) | 2 (13.3%) | 231 (20.5%) |
| Hospitalization Factors | ||
| Length of stay (days) | 7.0 (4.0, 14.0) | 5.00 (3.00, 8.00) |
| ICU stay during hospitalization | 8 (53.3%) | 203 (18.0%) |
| Geriatric/palliative involvement | 1 (6.7%) | 12 (1.1%) |
| Disposition | ||
| Home | 9 (60.0%) | 899 (79.4%) |
| Institution | 4 (26.7%) | 181 (16.0%) |
| Alive, unknown disposition | 2 (13.3%) | 52 (4.6%) |
| Hospital Characteristics | ||
| Teaching status | 7 (50.0%) | 520 (51.5%) |
| Small hospital size (< 200 beds) | 3 (21.4%) | 259 (25.2%) |
Values are n (%), unless otherwise indicated
Abbreviations: HF heart failure, HFrEF Heart failure with reduced ejection fraction, ICU Intensive care unit
Fig. 1.
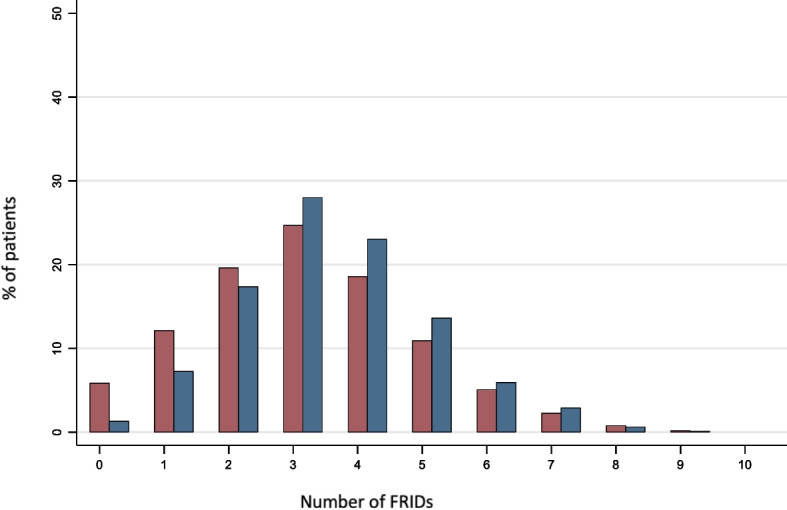
Frequency of FRID Counts at Admission (red) and Discharge (blue). FRIDs = fall risk-increasing drugs
The majority of FRIDs were CV in nature—on average, 82% of FRIDs were CV in nature at admission; and 88% of FRIDs were CV in nature at discharge. The proportion of individuals taking CV FRIDs increased from 92% at admission to 98% at hospital discharge (Table 3). The median count of CV FRIDs was 3 at admission and discharge (IQR: 2, 4). Between admission and discharge, 41% had at least one CV FRID added to their medication regimen. Antihypertensives were the most common class of CV FRID at both admission (88%) and discharge (93%). Common CV FRIDs included beta blockers (61% at admission, 75% at discharge), ACEi (36% at admission, 42% at discharge), and CCB (32% at admission, and 28% at discharge). The CV FRID with the greatest change in prevalence between admission and discharge was loop diuretics, which increased from 53% at admission to 72% at discharge (Table 3). These patterns were similar among those with HFrEF and HFpEF (Supplemental Table 4–5).
Table 3.
Prevalence of FRIDs use at hospital admission and discharge
| Admission | Discharge | Change | |
|---|---|---|---|
| All FRIDs | 1080 (94) | 1132 (99) | 5% |
| CV FRIDs | 1060 (92) | 1122 (98) | 6% |
| Antihypertensives | 1005 (88) | 1072 (93) | 5% |
| Beta blockers | 700 (61) | 863 (75) | 14% |
| ACE inhibitors | 411 (36) | 480 (42) | 6% |
| CCBs | 369 (32) | 325 (28) | -4% |
| Nitrates | 179 (16) | 250 (22) | 6% |
| Alpha blockers | 230 (20) | 215 (19) | 1% |
| ARBs | 228 (20) | 203 (18) | -2% |
| Vasodilators | 99 (9) | 152 (13) | 4% |
| Aldosterone antagonists | 81 (7) | 148 (13) | 6% |
| Thiazides | 183 (16) | 90 (8) | -8% |
| Potassium-sparing diuretics | 23 (2) | 8 (1) | -1% |
| Alpha agonists | 7 (1) | 9 (1) | - |
| Loop diuretics | 612 (53) | 829 (72) | 19% |
| Digoxin | 141 (12) | 185 (16) | 4% |
| Non-CV FRIDs | 369 (32) | 368 (32) | - |
| Antidepressants | 234 (20) | 243 (21) | 1% |
| SSRI | 157 (14) | 165 (14) | - |
| TCA | 46 (4) | 46 (4) | - |
| SNRI | 28 (2) | 30 (3) | 1% |
| Trazodone | 10 (1) | 12 (1) | - |
| NDRI | 8 (1) | 8 (1) | - |
| Benzodiazepines | 93 (8) | 87 (8) | - |
| Opioids | 97 (8) | 71 (6) | -2% |
| Antiepileptics | 30 (3) | 34 (3) | - |
| Antipsychotics | 19 (2) | 29 (2) | - |
| Atypical | 16 (1) | 24 (2) | 1% |
| Typical | 3 (0) | 5 (0) | - |
Abbreviations: FRIDs Fall risk-increasing drugs, CV Cardiovascular, ACEi Angiotensin-converting enzyme inhibitors, CCB Calcium channel blocker, ARB Angiotensin receptor blocker, Non-CV Non-cardiovascular, SSRI Selective serotonin reuptake inhibitor, TCA Tricyclic antidepressant, SNRI Serotonin and norepinephrine reuptake inhibitor, NDRI Norepinephrine and dopamine reuptake inhibitor
The prevalence of non-CV FRIDs was 32% at both admission and discharge. At admission, 23% took 1 non-CV FRID, 7% took 2 non-CV FRIDs, and 2% took 3 non-CV FRIDs. At discharge, 24% took 1 non-CV FRID, 7% took 2 non-CV FRIDs, and 2% took 3 non-CV FRIDs. Between admission and discharge, 8% had at least one non-CV FRID initiated. The most common class of non-CV FRID at both admission (20%) and discharge (21%) was antidepressants. The non-CV FRID with the greatest change between admission and discharge was opioids, which decreased from 8 to 6% (Table 3). These patterns were similar among those with HFrEF and HFpEF (Supplemental Tables 4–5).
At discharge, 54% of the cohort had an AHRQ score ≥ 6, indicating a high FRID burden (Fig. 2). The prevalence was comparable for HFpEF and HFrEF (Supplemental Fig. 3–4). In a multivariable analysis, factors most strongly associated with a high FRID burden at discharge were hypertension (PR: 1.41, 95% CI: 1.20, 1.65), mood disorder (PR: 1.24, 95% CI: 1.10, 1.38), and hyperpolypharmacy (PR: 1.88, 95% CI: 1.64, 2.14) (Table 4).
Fig. 2.
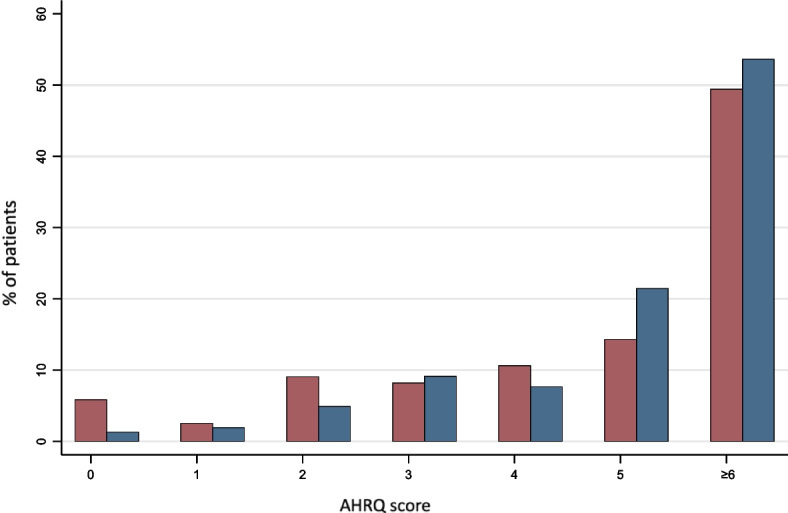
Frequency of AHRQ Fall Risk Score at Admission (red) and Discharge (blue). AHRQ = Agency for Healthcare Research and Quality
Table 4.
Predictors of high FRID burden at discharge
| PR | 95% CI | P value | |
|---|---|---|---|
| Age (per 10 years) | 0.92 | (0.86, 0.99) | 0.04 |
| Black | 1.11 | (0.99, 1.24) | 0.09 |
| Female | 1.02 | (0.91, 1.14) | 0.74 |
| Education less than high school | 0.98 | (0.86, 1.11) | 0.76 |
| Income less than $20,000 | 1.04 | (0.91, 1.19) | 0.57 |
| HFrEF | 1.03 | (0.91, 1.16) | 0.66 |
| Hypertension | 1.41 | (1.20, 1.65) | < 0.001 |
| Atrial fibrillation/atrial flutter | 1.00 | (0.88, 1.14) | 0.98 |
| Mood disorder | 1.24 | (1.10, 1.38) | < 0.001 |
| Osteoarthritis | 1.08 | (0.97, 1.21) | 0.18 |
| Rheumatoid arthritis | 0.73 | (0.47, 1.13) | 0.15 |
| Cancer | 1.07 | (0.95, 1.21) | 0.27 |
| Anticoagulation use | 0.94 | (0.83, 1.08) | 0.41 |
| Functional impairment | 1.09 | (0.98, 1.21) | 0.12 |
| Cognitive impairment | 1.13 | (0.96, 1.33) | 0.15 |
| History of falls | 1.04 | (0.92, 1.18) | 0.49 |
| Hyperpolypharmacy (at discharge) | 1.88 | (1.64, 2.14) | < 0.001 |
| Length of stay (per day) | 0.99 | (0.99, 1.01) | 0.50 |
| ICU stay during hospitalization | 0.97 | (0.84, 1.12) | 0.71 |
| Geriatric/palliative involvement | 0.85 | (0.43, 1.69) | 0.65 |
| Discharge to Facility | 0.89 | (0.76, 1.03) | 0.13 |
| Discharged Alive but unknown disposition | 1.20 | (0.97, 1.45) | 0.10 |
| Teaching hospital | 0.94 | (0.83, 1.06) | 0.32 |
| Small hospital (< 200 beds) | 1.05 | (0.92, 1.20) | 0.47 |
Abbreviations: PR Prevalence ratio, CI Confidence interval, HFrEF Heart failure with reduced ejection fraction, ICU Intensive care unit
Discussion
Older adults with HF are at a high risk of falls, which can lead to substantial physical, psychological, and social consequences. This supports the importance of understanding prescribing patterns of agents that can contribute to this risk (FRIDs). This study is the first to our knowledge to examine prescribing patterns of FRIDs in this population. From this study, we found that FRIDs are nearly universal among older adults hospitalized for HF, most take multiple FRIDs, and more than half of older adults with HF have a high FRID burden at hospital discharge. This work has important implications for the care of this population.
Our finding that FRIDs are nearly universal among older adults hospitalized for HF is important because FRIDs can potentially increase the risk for falls through their effects on the cardiovascular and central nervous systems [18, 19]. FRIDs may be especially problematic for older adults with HF, a subpopulation with an elevated risk for falls [2, 34, 35]. In a recent study of ambulatory patients with HF, 39% reported at least one fall in the prior year and 25% reported at least two falls in the prior year [36]. The risk of falls is further elevated following an HF hospitalization, as data from the Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial showed that the prevalence of falls prior to an HF hospitalization was 15%, and increased to 53% during the next 6 months [37]. Falls often lead to injuries and are associated with significant morbidity and mortality [38, 39]. Falls also have a significant psychological impact, leading to fear and anxiety surrounding future falls with related impairments in well-being and quality of life [39]. Given their highly prevalent use and potential impact on falls, FRIDs should become part of the usual lexicon for clinicians caring for older adults with HF.
FRIDs could be a potential target for deprescribing as a strategy to improve outcomes in older adults with HF. However, simply stopping or refraining from prescribing FRIDs may not be a simple decision. We found that the majority of FRIDs taken by adults with HF were CV in nature, and that many of these agents are recommended by clinical practice guidelines given their potential long-term benefit. For example, the most commonly initiated FRID on discharge was loop diuretics, which have a class I recommendation for treating HF [40]. Renin-angiotensin system inhibitors (beta-blockers, ACEi) are also beneficial for selected subtypes of HF. This creates a clinical conundrum. These agents are recommended given their potential to improve symptoms and/or long-term outcomes, but can increase the risk of falls via hypovolemia, hypotension, and/or bradycardia [41]. However, the risk of falls conferred by CV medications appears to be less than for non-CV drugs. Indeed, in a meta-analysis assessing medication use and falls in older adults, CV drugs (antihypertensives, diuretics) were less likely to cause falls compared to psychotropic drugs (sedatives/hypnotics, antidepressants, and benzodiazepines) [42]. As it may not be feasible to deprescribe CV medications given their clinical indication for HF, clinicians may instead consider regularly reviewing patient’s non-CV medications and evaluate their indication and side effect profile. On the whole, there is insufficient data to either support or refute the potential benefit of deprescribing FRIDs as a routine strategy to reduce fall risk in older adults. Therefore, decisions on how to address FRIDs use, such as potential dose optimization, in older adults with HF should be viewed as a preference-sensitive decision that requires shared decision-making and consideration of specific patient features (such as the presence of symptoms like hypotension or dizziness) [43]. Toward this end, several prescribing tools exist to aid clinicians in optimizing FRIDs use in the elderly, such as the STOPP/START (Screening Tool of Older Persons Prescriptions/Screening Tool to Alert to Right Treatment) tool, the Beers criteria, and the FORTA (Fit fOR The Aged) classification system [44–46]. Given their potential to reduce falls, they may be worth incorporating into routine clinical care of older adults with HF as part of a multifactorial falls prevention strategy [47, 48].
It may be important to consider FRID burden when making clinical decisions about medications. In this study, we used a tool for calculating FRID burden that is available on the AHRQ website. Although this tool requires further validation, it provides a convenient method for clinicians to quantify potential medication-related fall risk. It may be reasonable to use this tool as a starting point for a more comprehensive assessment to further assess overall fall risk in older adults with HF, especially among those with hyperpolypharmacy—a strong correlate for having a high FRIDs burden [49]. By identifying these patients, clinicians can then recommend select, targeted interventions to reduce falls risk. In addition, evaluating cognition, frailty, and gait may accordingly be an important strategy among those with hyperpolypharmacy given their association with falls [9, 50, 51]. At the very least, patients with a high FRID burden should be asked if they have a history of falls to identify risk, with subsequent incorporation into the risk and potential benefits of FRIDs.
Given the inherent risk of falls among older adults hospitalized for HF is in part attributable to the nearly universal use of FRIDs, it may be reasonable to routinely implement selected interventions for reducing the risk of falls. These interventions should include regular visits with primary care physicians to assess frailty status and gait speed as measures of functional ability. Clinicians can also provide patients practical tips to reduce falls risk, such as staying physically active, illuminating stairs and minimizing clutter in the household, wearing comfortable shoes, and using durable medical equipment such as canes or walkers. The World Falls Guidelines for falls prevention and management also emphasizes the importance of exercise programs including functional exercises (i.e.: sit-to-stand, stepping) and individualized strength training [52]. These exercises may be performed under the guidance and supervision of appropriately trained professionals, such as physiotherapists or kinesiologists, who can personalize them to the patients' functional status. Additional measures may involve employing home support services can help to create a safe environment, such as through the use of shower grab bars, handrails, and nonslip mats, and to implement quality home-fall hazard interventions. Occupational therapists may also help to assess environmental hazards and to determine the patients’ functional capacity in the context of their home environment. Regular vision assessments can also help to reduce fall risk [53]. Together, these measures are key to reduce falls and promote well-being, and thus should be part of a comprehensive care plan for many older adults with HF.
As outlined by a recent American Heart Association scientific statement, there is still much work to be done in the area of falls [1]. Few prospective studies have focused on identifying fall risk in adults with cardiovascular disease. Our study here serves as a foundation for future work examining the intersection of FRIDs and falls among older adults with HF, and for the development of individualized fall prevention interventions to meet the unique needs of this growing subpopulation of older adults living with HF.
This study has several strengths. First, REGARDS is a geographically diverse cohort of participants with HF treated in hospitals from all 48 contiguous United States. Therefore, this study has a high degree of generalizability specifically for older adults with HF. The availability of medical records was another strength of this study, as it allowed for detailed abstraction of participants’ medications and medical comorbidities at the time of hospitalization. Our study also has some limitations. First, we did not have sufficient data to calculate participant-level falls risk—it is likely that the risk of FRIDs is largely dependent on the participant-level falls risk. Accordingly, future work should focus on integrating FRID burden with participant-level falls risk. Second, we did not account for medication dosages or schedules, which likely impacts the medication-related risk for falls. Third, there is no universal list of FRIDs, and there is variation in the observed associations between various medications and falls. Different definitions could therefore lead to differing prevalence rates. We chose to examine a broad list of FRIDs based on prior comprehensive systematic reviews and meta-analyses [18, 19, 27–29]. Finally, while it is intuitive that higher scores would be associated with higher risk for falls, the AHRQ tool used in our study to quantify FRID burden has not yet been validated—this highlights yet another area ripe for future study.
Conclusion
In conclusion, our study showed that FRIDs were nearly universal among older adults hospitalized for HF, and more than half had a high FRID burden. Moreover, most of these FRIDs were CV in nature. This underscores the need for clinicians to consider fall risk in decision-making especially as it relates to medications.
Supplementary Information
Additional file 1: Supplemental Table 1. Baseline Characteristics According to FRIDs use at Admission. Supplemental Table 2. Baseline Characteristics According to FRIDs use at Discharge. Supplemental Table 3. Most Commonly Initiated and Discontinued FRIDs, Stratified by HF Subtype. Supplemental Table 4. Prevalence of FRIDs Use at Hospital Admission and Discharge Among Those With HFpEF. Supplemental Table 5. Prevalence of FRIDs Use at Hospital Admission and Discharge Among Those With HFrEF. Supplemental Figure 1. Frequency of FRID Counts for HFpEF. Supplemental Figure 2. Frequency of FRID Counts for HFrEF. Supplemental Figure 3. Frequency of AHRQ Fall Risk Score for HFpEF. Supplemental Figure 4. Frequency of AHRQ Fall Risk Score for HFrEF.
Acknowledgements
This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at https://www.uab.edu/soph/regardsstudy/. Additional funding was provided by National Heart, Lung, and Blood Institute (NHLBI) grant R01HL080477 and NIA grant R03AG056446. Representatives from the NIH did not have any role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation or approval of the manuscript.
Sponsor’s Role
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The National Institute on Aging, National Heart, Lung, and Blood Institute, and National Institute of Neurological Disorders and Stroke did not have a role in the design, methods, subject recruitment, data collections, analysis, or preparation of the manuscript.
Authors’ contributions
Dr. Liu and Dr. Goyal had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Liu, Goyal (informed by discussion with Hilmer). Acquisition, analysis, or interpretation of data: Nahid, Musse, Chen, Zullo, Kwak, Levitan, Safford. Drafting of the manuscript: Liu, Nahid, Musse, Goyal. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Nahid, Chen. Administrative, technical, or material support: Levitan, Safford. Study supervision: Lachs, Safford, Goyal.
Funding
This project was sponsored by cooperative agreement U01 NS041588 co-funded by the NINDS, NIA, NIH, and DHHS; NIH grant R01HL8077; and NIH grant R03AG056446. Dr. Goyal is supported by American Heart Association grant 20CDA35310455 and National Institute on Aging grant K76AG064428. Dr. Goyal was a member of the Junior Investigator Intensive Program of the US Deprescribing Research Network, which is funded by the National Institute on Aging (R24AG064025).
Availability of data and materials
Data was obtained from the REGARDS study, a private database. The datasets generated and/or analyzed during the current study are not publicly available, as the data includes characteristics that may compromise individual patient privacy; but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All experiments were performed in accordance with relevant guidelines and regulations (such as the Declaration of Helsinki). This study was reviewed and approved by the Weill Cornell Medicine Institutional Review Board and the University of Alabama-Birmingham Institutional Review Board. All REGARDS participants provided written informed consent and signed medical record release forms allowing REGARDS investigators to retrieve medical records for research purposes.
Consent for publication
Not applicable.
Competing interests
Dr. Goyal receives consulting fees from Sensorum Health; has received personal fees for medicolegal consulting related to heart failure; and has received honoraria from Akcea Therapeutics inc. Dr. Kwak receives consult fees from Endocrine & Diabetes Plus Clinic of Houston. Dr. Zullo receives grant funding paid directly to Brown University by Sanofi for research on the epidemiology of infections and vaccines in nursing home residents and infects. Dr. Levitan receives research support from Amgen, Inc. All other authors did not have any competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Denfeld QE, Turrise S, MacLaughlin EJ, et al. Preventing and managing falls in adults with cardiovascular disease: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2022;15(6):e000108. doi: 10.1161/HCQ.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 2.Tromp AM, Pluijm SM, Smit JH, Deeg DJ, Bouter LM, Lips P. Fall-risk screening test: a prospective study on predictors for falls in community-dwelling elderly. J Clin Epidemiol. 2001;54(8):837–844. doi: 10.1016/s0895-4356(01)00349-3. [DOI] [PubMed] [Google Scholar]
- 3.Healey F, Scobie S, Oliver D, Pryce A, Thomson R, Glampson B. Falls in English and Welsh hospitals: a national observational study based on retrospective analysis of 12 months of patient safety incident reports. Qual Saf Health Care. 2008;17(6):424–430. doi: 10.1136/qshc.2007.024695. [DOI] [PubMed] [Google Scholar]
- 4.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 5.Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16(1):158. doi: 10.1186/s12877-016-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman DE, Lipsitz LA. Syncope in the elderly. Cardiol Clin. 1997;15(2):295–311. doi: 10.1016/s0733-8651(05)70337-4. [DOI] [PubMed] [Google Scholar]
- 7.Chow GV, Marine JE, Fleg JL. Epidemiology of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28(4):539–553. doi: 10.1016/j.cger.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21(5):540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng MH, Chang SF. Frailty as a risk factor for falls among community dwelling people: evidence from a meta-analysis. J Nurs Scholarsh Off Publ Sigma Theta Tau Int Honor Soc Nurs. 2017;49(5):529–536. doi: 10.1111/jnu.12322. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney JE, Palta M, Johnson J, et al. Temporal association between hospitalization and rate of falls after discharge. Arch Intern Med. 2000;160(18):2788–2795. doi: 10.1001/archinte.160.18.2788. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK. The dilemma of delirium: Clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278–288. doi: 10.1016/0002-9343(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 12.Unlu O, Levitan EB, Reshetnyak E, et al. Polypharmacy in Older Adults Hospitalized for Heart Failure. Circ Heart Fail. 2020;13(11):e006977. doi: 10.1161/CIRCHEARTFAILURE.120.006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burt J, Elmore N, Campbell SM, Rodgers S, Avery AJ, Payne RA. Developing a measure of polypharmacy appropriateness in primary care: systematic review and expert consensus study. BMC Med. 2018;16(1):91. doi: 10.1186/s12916-018-1078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zia A, Kamaruzzaman SB, Tan MP. Polypharmacy and falls in older people: Balancing evidence-based medicine against falls risk. Postgrad Med. 2015;127(3):330–337. doi: 10.1080/00325481.2014.996112. [DOI] [PubMed] [Google Scholar]
- 15.Jaber D, Vargas F, Nguyen L, et al. Prescriptions for potentially inappropriate medications from the beers criteria among older adults hospitalized for heart failure. J Card Fail. 2022;28(6):906–915. doi: 10.1016/j.cardfail.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah A, Gandhi D, Srivastava S, Shah KJ, Mansukhani R. Heart failure: a class review of pharmacotherapy. Pharm Ther. 2017;42(7):464–472. [PMC free article] [PubMed] [Google Scholar]
- 17.Bell HT, Steinsbekk A, Granas AG. Factors influencing prescribing of fall-risk-increasing drugs to the elderly: a qualitative study. Scand J Prim Health Care. 2015;33(2):107–114. doi: 10.3109/02813432.2015.1041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries M, Seppala LJ, Daams JG, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: I. Cardiovascular Drugs. J Am Med Dir Assoc. 2018;19(4):371.e1-371.e9. doi: 10.1016/j.jamda.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Seppala LJ, Wermelink AMAT, de Vries M, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics J Am Med Dir Assoc. 2018;19(4):371.e11–371.e17. doi: 10.1016/j.jamda.2017.12.098. [DOI] [PubMed] [Google Scholar]
- 20.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 21.Sterling MR, Ringel JB, Pinheiro LC, et al. Social Determinants of Health and 30-Day Readmissions Among Adults Hospitalized for Heart Failure in the REGARDS Study. Circ Heart Fail. 2022;15(1):e008409. doi: 10.1161/CIRCHEARTFAILURE.121.008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal P, Kneifati-Hayek J, Archambault A, et al. Prescribing patterns of heart failure-exacerbating medications following a heart failure hospitalization. JACC Heart Fail. 2020;8(1):25–34. doi: 10.1016/j.jchf.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 25.2013 ACCF/AHA Guideline for the Management of Heart Failure | Circulation. Accessed August 13, 2021. https://www.ahajournals.org/doi/10.1161/cir.0b013e31829e8776
- 26.Butler J, Anker SD, Packer M. Redefining heart failure with a reduced ejection fraction. JAMA. 2019;322(18):1761–1762. doi: 10.1001/jama.2019.15600. [DOI] [PubMed] [Google Scholar]
- 27.Seppala LJ, van de Glind EMM, Daams JG, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others J Am Med Dir Assoc. 2018;19(4):372.e1–372.e8. doi: 10.1016/j.jamda.2017.12.099. [DOI] [PubMed] [Google Scholar]
- 28.Kahlaee HR, Latt MD, Schneider CR. Association between chronic or acute use of antihypertensive class of medications and falls in older adults. a systematic review and meta-analysis. Am J Hypertens. 2018;31(4):467–479. doi: 10.1093/ajh/hpx189. [DOI] [PubMed] [Google Scholar]
- 29.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47(1):40–50. doi: 10.1111/j.1532-5415.1999.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 30.NHANES - National Health and Nutrition Examination Survey Homepage. Published May 30, 2023. Accessed June 22, 2023. https://www.cdc.gov/nchs/nhanes/index.htm
- 31.Tool 3I: Medication Fall Risk Score and Evaluation Tools. Accessed August 13, 2021. http://www.ahrq.gov/patient-safety/settings/hospital/fall-prevention/toolkit/medication-risk-score.html
- 32.Beasley B, Patatanian E. Development and Implementation of a Pharmacy Fall Prevention Program. Hosp Pharm. 2009;44(12):1095–1102. doi: 10.1310/hpj4412-1095. [DOI] [Google Scholar]
- 33.Royston P, White IR. Multiple Imputation by Chained Equations (MICE): Implementation in Stata. J Stat Softw. 2011;45:1–20. doi: 10.18637/jss.v045.i04. [DOI] [Google Scholar]
- 34.Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: the health and retirement study. J Am Geriatr Soc. 2009;57(3):511–516. doi: 10.1111/j.1532-5415.2008.02150.x. [DOI] [PubMed] [Google Scholar]
- 35.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337(18):1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 36.Denfeld QE, Goodlin S, Abedalweli R, et al. Frequency and Predictors of Falls Among Adults With Heart Failure: A Prospective Study. J Card Fail. Published online October 12, 2022. 10.1016/j.cardfail.2022.09.011 [DOI] [PMC free article] [PubMed]
- 37.Mentz RJ, Whellan DJ, Reeves GR, et al. Rehabilitation Intervention in Older Patients With Acute Heart Failure With Preserved Versus Reduced Ejection Fraction. JACC Heart Fail. 2021;9(10):747–757. doi: 10.1016/j.jchf.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaishya R, Vaish A. Falls in older adults are serious. Indian J Orthop. 2020;54(1):69–74. doi: 10.1007/s43465-019-00037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartholt KA, van Beeck EF, Polinder S, et al. Societal consequences of falls in the older population: injuries, healthcare costs, and long-term reduced quality of life. J Trauma. 2011;71(3):748–753. doi: 10.1097/TA.0b013e3181f6f5e5. [DOI] [PubMed] [Google Scholar]
- 40.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 41.Preventing and Managing Falls in Adults With Cardiovascular Disease: A Scientific Statement From the American Heart Association. 10.1161/HCQ.0000000000000108 [DOI] [PubMed]
- 42.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Negm A, Peters R, Wong EKC, Holbrook A. Deprescribing fall-risk increasing drugs (FRIDs) for the prevention of falls and fall-related complications: a systematic review and meta-analysis. BMJ Open. 2021;11(2):e035978. doi: 10.1136/bmjopen-2019-035978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. 10.1111/jgs.13702 [DOI] [PubMed]
- 46.Pazan F, Wehling M. The FORTA (Fit fOR The Aged) App as a clinical tool to optimize complex medications in older people. J Am Med Dir Assoc. 2017;18(10):893. doi: 10.1016/j.jamda.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Michalek C, Wehling M, Schlitzer J, Frohnhofen H. Effects of “Fit fOR The Aged” (FORTA) on pharmacotherapy and clinical endpoints–a pilot randomized controlled study. Eur J Clin Pharmacol. 2014;70(10):1261–1267. doi: 10.1007/s00228-014-1731-9. [DOI] [PubMed] [Google Scholar]
- 48.Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc. 2014;62(9):1658–1665. doi: 10.1111/jgs.12993. [DOI] [PubMed] [Google Scholar]
- 49.Gorodeski EZ, Goyal P, Hummel SL, et al. Domain management approach to heart failure in the geriatric patient: present and future. J Am Coll Cardiol. 2018;71(17):1921–1936. doi: 10.1016/j.jacc.2018.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Racey M, Markle-Reid M, Fitzpatrick-Lewis D, et al. Fall prevention in community-dwelling adults with mild to moderate cognitive impairment: a systematic review and meta-analysis. BMC Geriatr. 2021;21(1):689. doi: 10.1186/s12877-021-02641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64A(8):896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montero-Odasso M, van der Velde N, Martin FC, et al. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing. 2022;51(9):afac205. doi: 10.1093/ageing/afac205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of Interventions for Preventing Falls in Older Adults: A Systematic Review and Meta-analysis. JAMA. 2017;318(17):1687–1699. doi: 10.1001/jama.2017.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Baseline Characteristics According to FRIDs use at Admission. Supplemental Table 2. Baseline Characteristics According to FRIDs use at Discharge. Supplemental Table 3. Most Commonly Initiated and Discontinued FRIDs, Stratified by HF Subtype. Supplemental Table 4. Prevalence of FRIDs Use at Hospital Admission and Discharge Among Those With HFpEF. Supplemental Table 5. Prevalence of FRIDs Use at Hospital Admission and Discharge Among Those With HFrEF. Supplemental Figure 1. Frequency of FRID Counts for HFpEF. Supplemental Figure 2. Frequency of FRID Counts for HFrEF. Supplemental Figure 3. Frequency of AHRQ Fall Risk Score for HFpEF. Supplemental Figure 4. Frequency of AHRQ Fall Risk Score for HFrEF.
Data Availability Statement
Data was obtained from the REGARDS study, a private database. The datasets generated and/or analyzed during the current study are not publicly available, as the data includes characteristics that may compromise individual patient privacy; but are available from the corresponding author on reasonable request.


