Figure 5.
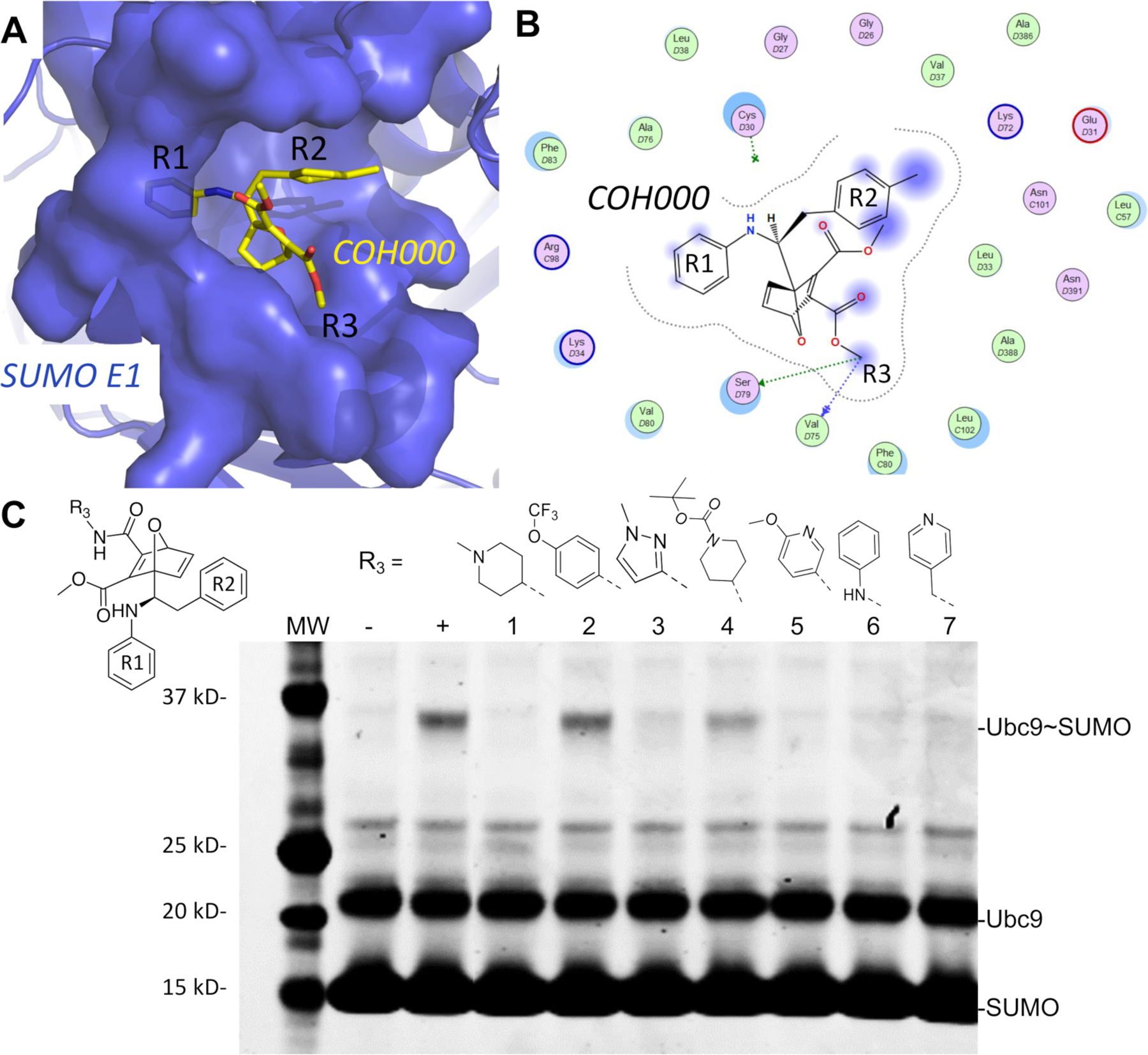
The lowest energy non-covalent bound conformation of the SUMO E1-COH000 complex identfied from LiGaMD simulations were supported by SAR of the inhibitor analogs. (A) The Non-covalent Bound” (NC-B) conformation of COH000 in the SUMO E1 protein pocket. (B) 2D LigPlot representation of the binding pocket residues of SUMO E1 that interact with COH000 as observed in Fig. 4D. Blue filled circles on COH000 represent solvent accessibility with larger circles representing greater solvent accessibility. Polar and non-polar residues are colored in pink and green circles, respectively. Acidic and basic residues have a red and blue circle border, respectively. (C) Analogs with larger R3 groups exhibit lower inhibitory effects, which is consistent with the steric restriction of the region interacting with this site of the compound as shown in (A). “−“ and “+” represent Ubc9-SUMO thioester formation catalyzed by E1, in the absence and presence of ATP, respectively, as controls. All reactions with compounds were conducted in the presence of ATP and at the same compound molar concentration, and lack of Ubc9-SUMO thioester formation bands indicates inhibition and the presence of Ubc9-SUMO thioester formation bands indicates lack of inhibition.
