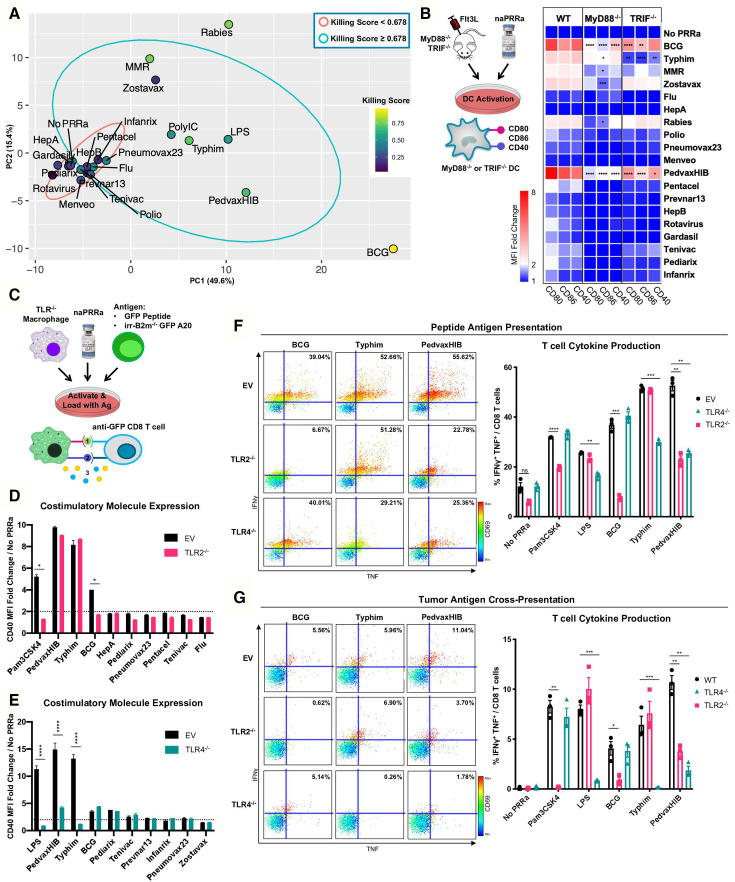
Figure 3.
Individual naPRRa possess unique activation profiles and function through distinct immunological mechanisms. (A) PCA was performed with 155 phenotypic markers on murine immune cells activated with naPRRa as loading variables. Graph of PC1 and PC2 is shown. naPRRa are shaded based on potency of cytotoxic T-cell response (Killing Score). Ellipses are drawn representing naPRRa that induce significantly stronger T-cell cytotoxicity than the No PRRa condition (blue) versus naPRRa that are not superior in cytotoxic T-cell induction compared with the No PRRa condition. (B) DC activation assay using TRIF-/- and MyD88-/- mice. MyD88-/- DCs become less activated with Rabies, Zostavax, and MRR. BCG, Typhim, and PedvaxHIB-mediated activation is suppressed in both TRIF-/- and MyD88-/- DCs. N=3 mice for each genotype. Statistical significance was calculated by two-way ANOVA with Dunnett’s multiple comparison test, comparing to wild type (WT) DCs. (c) Schema of the TLR-/- RAW cell activation and cross-priming assays. WT and TLR-/- RAW cells were activated with vaccine naPRRa, and flow cytometry was used to compare CD40 upregulation in WT vs TLR2-/- (D) and TLR4-/- (E). Statistical significance was calculated by t test. (F, G) TLR-/- RAW cells were cocultured with GFP peptide or XRT B2m-/- GFP-A20 to acquire antigen, activated with naPRRa, then cocultured with purified anti-GFP CD8 T-cells. Priming and cross-priming was measured as CD8 T-cell activation by flow cytometry. Representative scatter plots showing that naPRRa promote anti-GFP CD8 T-cell peptide antigen priming (F) or cross-priming with tumor-derived antigen (G) by targeting TLR2 and TLR4 on RAW cells. Statistical significance was calculated by t test. ANOVA, analysis of variance; DC, dendritic cells; PCA, principal component analysis; PRRa, pattern recognition receptor agonists.