Figure 1.
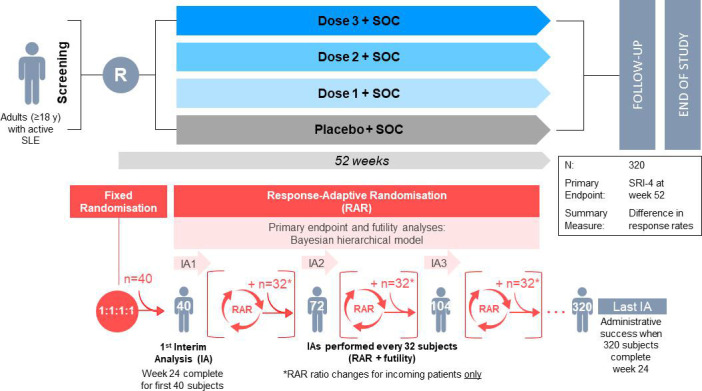
Adaptive trial schema and innovative design elements of the phase IIb studies for rozibafusp alfa and efavaleukin alfa in SLE. Innovative elements: 1. Study implements response-adaptive randomisation; 2. Multiple interim analyses that evaluate for futility and one efficacy evaluation (primary); 3. Primary endpoint (SRI-4 response at week 52) and futility analyses are evaluated using a Bayesian hierarchical model comparing the three dose levels to placebo. SOC, standard of care; SRI, Systemic Lupus Erythematosus Responder Index.
