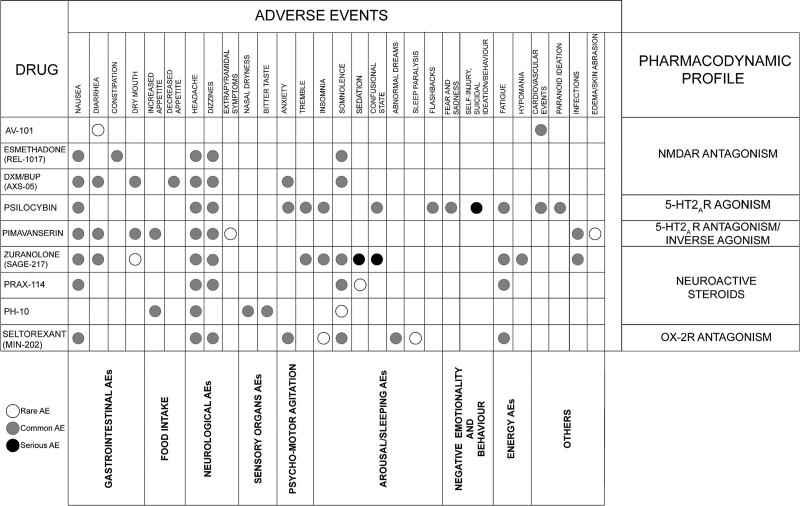
Fig. 2.
Description of adverse events of novel antidepressant drugs. Common adverse events were defined as those having a frequency ≥5%, while rare adverse events as those with a frequency <5%. Serious adverse events are defined as any adverse event causing clinically significant disability and incapacity or immediately life-threatening. BUP, bupropion; DXM, dextromethorphan; NMDAR, n-methyl-d-aspartate receptor; OX-2R, orexin type 2 receptor; R, receptor.