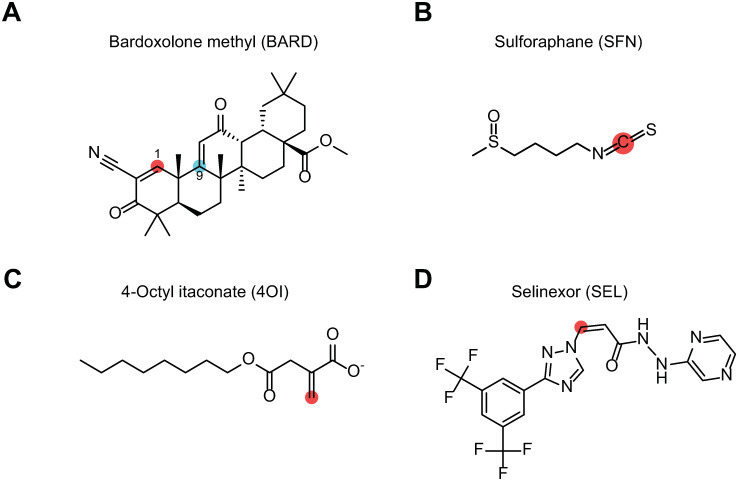
Fig 1. Chemical structures of the four compounds used.
The reactive electrophilic carbon atoms that can potentially undergo Michael addition from nucleophilic targets are highlighted in red or blue. A. Bardoxolone methyl (BARD) is unique in that it has two reactive carbon atoms at positions 1 (red) and 9 (blue). B. Sulforaphane (SFN). C. 4-Octyl itaconate (4OI). D. Selinexor (SEL). This bona fide XPO1 inhibitor is not known to be an NRF2 agonist, but also possesses one electrophilic double bond.