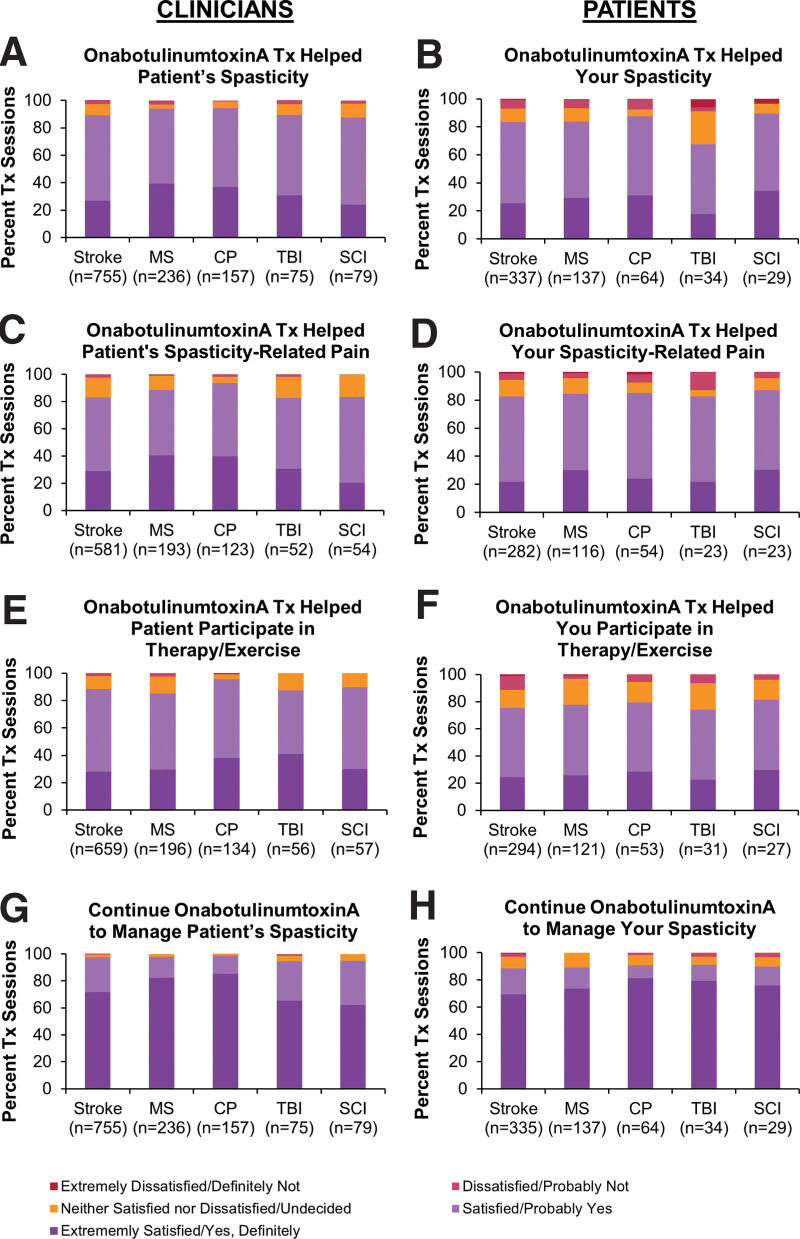
Figure 3.
Clinician (A, C, E, G)- and patient (B, D, F, H)-reported satisfaction with the previous onabotulinumtoxinA treatment for upper and/or lower limb spasticity. Questions were (A) “How satisfied, on average, are you that the most recent Botox treatment has helped to manage your patient’s spasticity symptoms?” (B) “How satisfied are you that your most recent Botox treatment has helped your spasticity?” (C) “How satisfied are you that the most recent Botox treatment has helped to manage the pain associated with your patient’s spasticity?” (D) “How satisfied are you that your most recent Botox treatment has helped your spasticity-related pain?” (E) “How satisfied are you that the most recent Botox treatment has helped with your patient’s ability to participate in physiotherapy, occupational therapy (PT/OT) and/or an exercise program?” (F) “How satisfied are you that your most recent Botox treatment has helped with your ability to participate in physiotherapy, occupation therapy (PT/OT), and/or home exercise?” (G) “Taking everything into consideration, would you continue to use Botox to manage your patient’s spasticity?” (H) “Taking everything into consideration, would you continue to use Botox to treat your spasticity?” Respondents were given a choice of 5 answers ranging from “extremely satisfied” to “extremely dissatisfied” for A to F and from “yes, definitely” to “definitely not” for G and H. Figure is reprinted with permission from “High clinician- and patient-reported satisfaction with individualized onabotulinumtoxinA treatment for spasticity across several etiologies from the ASPIRE study” by Francisco GE, licensed under CC BY-NC-ND 4.0. CP = cerebral palsy, MS = multiple sclerosis, n = number of treatment sessions, OT = occupational therapy; PT = physical therapy; SCI = spinal cord injury; TBI = traumatic brain injury, Tx = treatment session.