Abstract
Objective To assess the viability of bovine ovarian tissue after cryopreservation through either slow freezing or vitrification, and to compare it to that of control tissue by performing morphological analyses.
Methods The study included 20 bovine ovarian cortex fragments that were divided into control, vitrification, and slow freezing groups. Each group consisted of four fragments of the same ovary, two fixed without cultivation, and two fixed with cultivation. Tissues were evaluated based on follicular morphology immediately after heating and after 7 days of culture, and compared with the control group.
Results A total of 240 fragments were analyzed, generating a sample of 1,344 follicles without cultivation and 552 with cultivation. When the non-cultivated samples were classified as non-atretic follicles, 572 were found in the control group, 289 in the vitrification group, and 373 in the slow freezing group, showing no significant differences. When classified as atretic, 46 follicles were found in the control group, 23 in the vitrification group, and 41 in the slow freezing group, also showing no statistical difference. In the post-culture sample, an evolution of the follicular stages could be observed. This finding was important to support that the follicles considered non-atretic in the non-cultivated group were actually viable in the morphological evaluation.
Conclusion With no differences between the protocols, vitrification was shown to be an advanced and alternative method for patients who will undergo treatments that carry the risk of ovarian failure, as the method is less expensive, faster, and more adaptable to laboratory routine.
Keywords: ovarian tissue, vitrification, slow freezing, fertility
Abstract
Resumo
Objetivo avaliar a viabilidade do tecido ovariano bovino após a criopreservação, utilizando congelamento lento e vitrificação, e comparando com o tecido controle por meio de análises morfológicas.
Métodos o estudo incluiu fragmentos de córtex de vinte ovários bovinos divididos em grupos controle, vitrificação e congelamento lento. Cada grupo foi composto por quatro fragmentos do mesmo ovário, sendo dois fragmentos fixados sem cultivo e dois fragmentos fixados pós-cultivo. Os tecidos foram avaliados pela morfologia folicular logo após o aquecimento e após sete dias de cultivo, e comparados com o grupo controle.
Resultados um total de 240 fragmentos foi analisado, gerando uma amostra de 1.344 folículos sem cultivo e 552 pós-cultivo. Quando a amostra sem cultivo teve seus folículos agrupados em não atrésicos, obtivemos 572 no grupo controle, 289 no vitrificação, e 373 no congelamento lento, não apresentando diferença estatística. Quando agrupados em atrésicos, o grupo controle apresentou 46 folículos, o vitrificação, 23, e o congelamento lento, 41, não apresentando também diferença estatística. Na amostra pós-cultivo, podemos observar uma evolução dos estágios foliculares: esse achado foi importante para sustentar que os folículos considerados não atrésicos na avaliação morfológica sem cultivo estavam realmente viáveis.
Conclusão não havendo diferenças entre os protocolos, a vitrificação se mostra um avanço e um método alternativo para pacientes que irão se submeter a tratamentos que podem levar a uma falência ovariana, uma vez que a metodologia é mais barata, mais rápida e mais bem adaptável a uma rotina de um laboratório.
Palavras-chaves: tecido ovariano, vitrificação, congelamento lento, fertilidade
Introduction
Fertility preservation is aimed at increasing the probability of men and women having biological children after their reproductive abilities are impaired due to several causes.1 The main treatments are those used in oncology (surgery, chemotherapy, or radiotherapy).2 However, with early diagnosis and the newly available therapies, most of these patients will have a good chance of cure or long survival,3 but may have impaired fertility.
Techniques for the cryopreservation of embryos, oocytes and ovarian tissue have been used to preserve female fertility.4 The use of oocytes and embryos is an alternative that has been established and standardized.5 However, its application is not without limitations.6 The main limitations are the time required for the application, the high doses of hormones used during ovarian stimulation, and the impossibility of use in children and adolescents.7 8 Considering these difficulties, procedures for ovarian tissue cryopreservation that can be used in pre-pubescent young patients and in those who require immediate treatment, without time for ovarian stimulation, should be developed. If the techniques evolve, results may be better than the current ones with oocyte and embryo freezing,9 as cryopreserved fragments could be transplanted back into the patients not only to achieve pregnancy, but also to restore hormone production.
Sixty births have been reported so far after retransplantation of cryopreserved ovarian tissue, and other pregnancies are under way.10 Many questions still need to be clarified for the procedure to progress from the experimental phase and become part of the routine treatment available. The main points still to be clarified are: knowledge of the real effectiveness of the method; correction of ischemia in the retransplantation site; and definition of the best laboratory protocol for cryopreservation.11
The most used methodology in early studies was the slow freezing technique, which has been responsible for most births.12 Recently, however, the vitrification method has arisen, increasing interest because of the good results obtained with the vitrification of oocytes and embryos.13 14 On the other hand, it remains unclear whether the method is also superior for preserving ovarian tissue. Many efforts have been made to compare slow freezing and vitrification. However, the results are still quite controversial. Some of the reasons for these discrepant results may be a reflection of: the number of existing protocols; the sizes of the cryopreserved fragments; the different types and concentrations of the cryoprotectants used; the heterogeneity of the follicular pool; and the diverse end points used in the analyses in the different studies that favored either slow freezing15 or vitrification,16 or suggested that both methods are the same.17
In order to provide answers to these questions, the present study aimed to compare the viability of bovine fresh ovarian tissue with that of ovarian tissue after cryopreservation using two methods, namely slow freezing and vitrification, by analyzing morphology and follicular density.
Methods
A prospective study was conducted using a convenience sample of 20 ovaries of cows slaughtered for meat in an abattoir, collected over 5 days, with the extraction of four ovaries per day. The ovaries were collected immediately after slaughter by previously trained staff, and forwarded to the research laboratory of the Pró-Criar Medicina Reprodutiva in a plastic container with a transport solution cooled to 4°C (glucose serum plus 10 µg/ml gentamicin, Sigma-Aldrich, St. Louis, Missouri, US). After arriving at the laboratory, they were washed with distilled water. After macroscopic analysis, the ovaries that were oval and flattened side to side were considered normal and used in the experiment. The study was conducted after approval by the Ethics and Animal Use Committee of the Faculdade de Ciências Médicas de Minas Gerais, having met all requirements.
In order to process the ovaries, scalpels and curved scissors were used. First, the ovaries were cut in half, and then the cortex was separated from the medulla with a tissue slicer. The medulla was discarded, and the cortex was placed in the manipulation medium (α-MEM, Irvine Scientific, Irvine, California, US) supplemented with 5% synthetic serum substitute (99193, Irvine) and gentamicin 10 µg/ml (G1264, Sigma-Aldrich) heated at 37°C. The cortex was cut into slices of 4 × 1 × 0.5 mm (length × width × diameter), and then into several fragments of ∼ 1 × 1 × 0.5 mm.
Four fragments from each animal were obtained and divided into three groups (control, slow freezing, and vitrification). From the divided groups, 2 fragments of the control group were left overnight in 4% formalin, and the other 2 were placed in culture for 7 days for posterior fixation in 4% formalin. The respective four fragments of the vitrification and slow freezing groups were first frozen according to the techniques proposed. After heating, 2 fragments were directly placed in 4% formalin, and the other 2 were placed in culture for 7 days for posterior fixation in 4% formalin. This procedure was repeated for all of the 20 ovaries used in the study (Fig. 1).
Fig. 1.
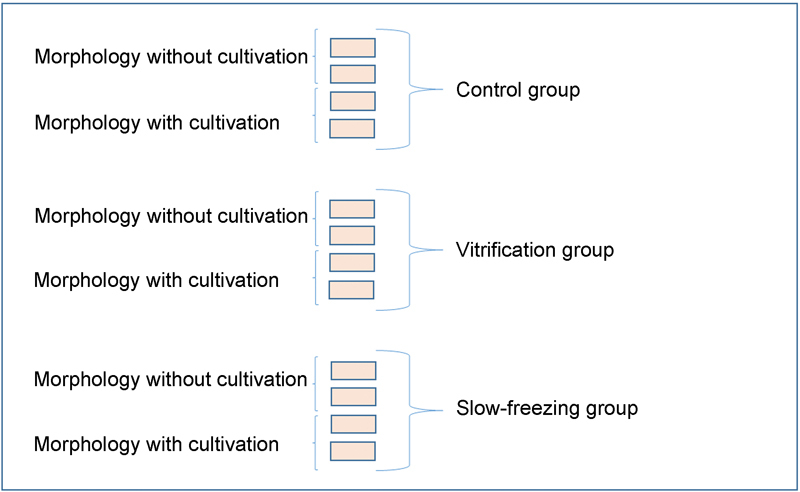
Division of ovarian tissue fragments into groups and their outcomes.
For the fixation of the fragments and subsequent morphological analysis, petri dishes (Falcon BD, New York, USA) containing 3 mL of 4% formalin were used. The fragments remained in the solution overnight (±15 hours), and then were placed in Eppendorf tubes containing 1 mL of 70% ethanol and stored in a refrigerator (±4°C).
For the 7-day cultivation of the fragments, 48-well microplates were used (Falcon BD, New York, USA). They contained: 300 μL per well of culture medium (α-MEM supplemented with 3 ng/mL follicle-stimulating hormone [F2293, Sigma-Aldrich]); 0.3% serum substitute supplement (SSS; 99193, Irvine); 5 μg/mL insulin (I2643, Sigma-Aldrich); 5 μL/mL transferrin (T8158, Sigma-Aldrich); 5 ng/mL sodium selenite (S5261, Sigma-Aldrich); 1 mg/mL bovine fetuin (F6131, Sigma-Aldrich); and 10 mg/ml gentamycin (G1264, Sigma-Aldrich). In order to maintain moisture, 300 μL of distilled water was added to the peripheral wells. On days D1, D4, and D6, 150 μL of the culture medium in each well that contained a fragment was stored in an Eppendorf tube, and 150 μL of fresh culture medium was added to each well to maintain tissue viability. The Eppendorf tubes containing the culture medium were stored in a freezer for future hormonal analysis. At the end of D7, the fragments of ovarian tissue were placed in an Eppendorf tube containing 1 ml of 70% ethanol for fixing and subsequent morphological analysis.
In the vitrification procedure, the samples were vitrified using the method described by Yeoman et al18 and Ting et al.19 The fragment samples were initially and sequentially balanced in solutions containing: 1.2 M glycerol (10% glycerol v/v; G2025, Sigma-Aldrich) for 3 minutes; 1.2 M glycerol (G2025, Sigma-Aldrich); ethylene glycol + 3.6 M (10% glycerol + 20% ethylene glycol; 102466, Sigma-Aldrich) for 3 minutes; and 3 M glycerol (G2025, Sigma-Aldrich) + 4.5 M ethylene glycol (25% glycerol + 25% ethylene glycol; 102466, Sigma-Aldrich) for 1 minute. The whole process was performed at room temperature. After emersion in the last solution, the fragments were placed individually in aluminum foil (8 × 4 mm2), and immediately immersed in liquid nitrogen, transferred to cryovials, and stored at −196°C until it was time for thawing. For heating, the cryotubes were removed from liquid nitrogen, and the fragments were immediately placed individually for 5 minutes in solutions containing 0.5 M sucrose (S1888, Sigma-Aldrich), 0.25 M sucrose (S1888, Sigma-Aldrich), 0.125 M sucrose (S1888, Sigma-Aldrich) in equilibrium, and finally placed 2 times in an equilibrium solution for 10 minutes. After heating, the specimens were kept in the equilibrium solution supplemented with 15% (v/v), SSS (99193, Irvine), and 29-mg/mL ascorbic acid phosphate (A4403, Sigma-Aldrich). Two of the heated fragments from each cow were placed in 4% formalin, and the other 2 were kept in culture for 7 days for subsequent fixation in 4% formalin and morphological assessment of viability after culture.
For slow freezing, samples were cryopreserved using the slow freezing method currently used by the American Medical Cooperative of Oncofertility Consortium (National Physicians Cooperative of the Oncofertility Consortium).20 The ovarian tissue was individually placed in a cryovial with a solution containing 1 ml of 1.5 M ethylene glycol (102466, Sigma-Aldrich) and 1.0 M sucrose (S1888, Sigma-Aldrich). For freezing, an equipment Freeze Control Cl 8000 (Australia) with a programmed freezing ramp was used, following the pattern of 2°C/min to −7°C, and kept at this temperature for 20 minutes. The seeding was performed after the first 10 minutes at −7°C, followed by 0.3°C/min to −30°C and free fall to −60°C. After ramp freezing, the fragments were stored in liquid nitrogen (−196°C).
For thawing, the cryotubes were kept at room temperature for 1 minute, and then kept at 37°C in a water bath for 2 minutes. After this period, the specimens were placed in petri dishes containing 1.0 M ethylene glycol for 10 minutes, and 0.5 M ethylene glycol for 10 minutes, and finally in a manipulation medium for 5 minutes. Two of the heated fragments from each animal were placed in 4% formalin, and the other 2 were kept in culture for 7 days for subsequent fixation in 4% formaldehyde and morphological assessment of viability after culture.
For the morphological evaluation of all the ovarian tissue fragments, histological slides were prepared and processed for analysis in the histology laboratory of the Faculdade de Ciências Médicas de Minas Gerais. They were stained with hematoxylin and eosin, and then analyzed using a light microscope at ×200 magnification to observe follicular structures and stromal cells.
The samples fixed in 4% formalin were embedded in paraffin, cut, and stained with hematoxylin and eosin. Sections of 4-µm width were cut. Of every six sections, the second fragment on the slide was evaluated in relation to follicle number, morphology, development stage, and presence or absence of oocytes (Fig. 2).
Fig. 2.
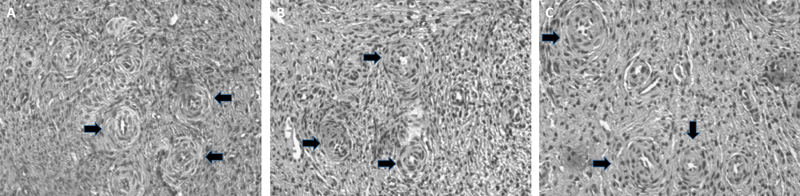
(A) Bovine ovarian tissue from the control group. (B) Ovarian tissue from the vitrification group. (C) Ovarian tissue from the slow freezing group. Arrows indicate some of the identified follicles in the tissue. Source: authors' images.
The numbers of primordial, transient, primary, secondary, antral, and atretic follicles were counted. Follicular structures in direct contact with the oocyte and the granulosa cells around them were considered normal, as well as the contact between the granulosa cells. These should not present enlarged intercellular spaces between them, cytoplasmic contraction, and nuclear pyknosis in granulosa cells and oocytes. Vacuolated oocyte nuclei were considered abnormal.
The statistical analysis consisted of absolute and relative frequencies for categorical variables and mean ± standard deviation (SD) for continuous variables. The comparison of the two proportions was performed using the Pearson Chi-square proportions test. Analyses were performed by using the free software R version 3.1.3 (Boston, MA, USA). The significance level was 5%.
The statistical power of the sample was 98%. The calculation was based on the size of the analyzed sample and on the study's objective of comparing the two techniques.
Results
Of the 240 ovarian tissue fragments from the 20 ovaries of cows, 120 non-cultivated analyzed fragments provided a sample of 1,344 follicles, which were the subject of our morphological analysis without culture. Of these follicles, 618 (46%) were evaluated as controls, 312 (23%) using the vitrification method, and 414 (31%) using the slow freezing method. Regarding their developmental stages, 822 follicles (61%) were in the primordial stage; 145 (11%) were in the transient stage; 201 (15%) were primary follicles; 38 (3%) were secondary follicles; 28 (2%) were in the antral stage; and 110 (8%) were in the atretic stage.
Among the cultivated samples, 120 ovarian tissue fragments provided a sample of 552 follicles, which were the subject of our morphological analysis with cultivation. Of these follicles, 163 (30%) were evaluated as controls, 254 (46%) using the vitrification method, and 135 (24%) using the slow freezing method. Regarding their developmental stages, 258 follicles (48%) were in the primordial stage; 86 (15%) were in the transient stage; 91 (16%) were primary follicles; 29 (5%) were secondary follicles; 10 (2%) were in the antral stage, and 78 (14%) were in the atretic stage.
For non-cultivated morphology, when the follicles were classified as non-atretic (follicles at any stage of development that were viable according to the integrity of the membrane and spacing of the cells), 572 follicles (92.6%) were found in the control group; 289 (92.6%) in the vitrification group; and 373 (90.1%) in the slow-freezing group. When classified as atretic (follicles in all stages of development presenting cell degeneration and spacing), 46 follicles (7.6%) were obtained from the control group; 23 (7.4%) from the vitrification group; and 41 (9.9%) from the slow freezing group. No significant difference was observed between the groups (p > 0.05). In the comparison of the results of the stage of development of the follicles counted, the proportion of follicles in the transitory stage was significantly greater in the control group than in the slow freezing and vitrification groups (p < 0.05). No significant differences were observed regarding other groups and stages (Table 1).
Table 1. Distribution of the number of follicles in each developmental phase in the uncultivated samples in each group.
| Stage | Control (n = 618) |
Vitrification (n = 312) |
Slow freezing (n = 414) |
|---|---|---|---|
| n | n | n | |
| Primordial | 362 | 203 | 257 |
| Transient | 96£ € | 15£ | 34€ |
| Primary | 79 | 53 | 69 |
| Secondary | 16 | 14 | 8 |
| Antral | 19 | 4 | 5 |
| Atretic | 46 | 23 | 41 |
p < 0.05, in the comparison test of proportions among the groups.
p < 0.05, in the comparison test of proportions among the groups.
In the comparison between the morphologies with and without culture, a significant decrease in the proportion of follicles in the primary stage was observed among the samples that were cultivated in all groups. In the control group, increases in the proportions of secondary and atretic follicles among the cultivated samples were observed. In both the vitrification and slow freezing groups, a significantly higher number of follicles in the transitional stage was found in the samples with culture (Table 2).
Table 2. Distribution of the number of follicles in each developmental phase in the samples with and without cultivation in each group.
| Developmental stage | Control | Vitrification | Slow freezing | |||
|---|---|---|---|---|---|---|
| Without cultivation (n = 618) |
With cultivation (n = 163) |
Without cultivation (n = 312) |
With cultivation (n = 254) |
Without cultivation (n = 414) |
With cultivation (n = 135) |
|
| n | n | n | n | n | n | |
| Primordial | 362* | 65* | 203£ | 133£ | 257€ | 60€ |
| Transient | 96 | 33 | 15£ | 31£ | 34€ | 22€ |
| Primary | 79 | 15 | 53 | 47 | 69 | 29 |
| Secondary | 16* | 10* | 14 | 14 | 8 | 5 |
| Antral | 19 | 1 | 4 | 6 | 5 | 3 |
| Atretic | 46* | 39* | 23 | 23 | 41 | 16 |
p < 0.05, in the comparison test between fresh and post-cultivation proportions in each evaluation group.
p < 0.05, in the comparison test between fresh and post-cultivation proportions in each evaluation group.
p < 0.05, in the comparison test between fresh and post-cultivation proportions in each evaluation group.
Discussion
In order to define the best protocol for ovarian tissue cryopreservation, using human live births after retransplantation would be ideal as the outcome. These studies present great difficulties due to the complexity and variety of factors that influence the occurrence of pregnancy.
Intermediate outcomes are adopted in the development of various procedures and treatments to enable initial assessments. Follicular morphology, hormone production, and proliferation of tissue markers have been used to evaluate the viability of ovarian tissue,21 as well as a bovine model, due to the availability of the material for use in studies.22
Cryopreservation of the ovarian tissue, by either slow freezing or vitrification, is a challenge for cryobiologists, given the composition of the tissue, which includes different cell types such as: stromal cells; follicles in different stages of development; granulosa and theca cells; and blood vessels and nerves, which may require different conditions for the cryopreservation process. This cell variation hinders the diffusion of water and cryoprotectors,23 compromising the viability of each cell compartment after cryopreservation.24 Unlike oocytes, which have a single cell structure and embryos which have a large number of cells with their similar structures.
In order to assess which protocol maintains better ovarian tissue viability, a morphological analysis of the conditions of the cryopreserved fragments was conducted using both methods; the groups were then compared with each other and with the control group. The fragments were obtained from the same ovarian tissue from each animal and processed in parallel using both protocols (vitrification and slow freezing). Morphological comparison was chosen because it has been proven effective for assessing viability in several studies.25 26
In our study, 46% of the follicles analyzed were present in the control group, which showed a great loss of follicles after thawing. Variations may probably occur because of the size of the fragments, type and duration of exposure to cryoprotectants, and the heterogeneity of ovarian tissues, when compared with the amount of follicles and their stages. This makes an appropriate entry and exit for cryoprotectants in several follicular types. Due to this loss of follicular pool, improving the protocols is even more necessary for the fertility preservation through ovarian tissue cryopreservation, this being really effective and beneficial to patients.
Most of the follicles found in our study were in the primordial stage (61%). Data are in agreement with previous findings, which showed that the proportion of primordial follicles was higher than that of other stages in bovine ovaries.27
Kim et al28 suggested that the pool of primordial and primary follicles would be responsible for the resumption of hormone production and the generation of pregnancy after transplanting ovarian tissue back to the patient. However, the quantity and diversity of follicles necessary for a successful retransplantation is still a factor to be determined.
The data from this study demonstrate the maintenance of the heterogeneity of ovarian tissue and survival of a large number of primordial follicles. Moreover, the results suggest that after cryopreservation by any of the methods, the transplanted fragments would be able to resume ovarian function.
In order to prevent the reintroduction of neoplastic cells in the patient, in vitro maturation of primordial follicles has been proposed.29 30 31 For the fertilization of oocytes in the laboratory, the technique is still being developed. For the isolation and subsequent maturation of these follicles, secondary follicles are used. In our study, of the follicles at this stage, 2.6% were in the control group, 4.5% were in the vitrification group, and 1.9% were in the slow freezing group. Although these numbers are not significant, they may suggest that the vitrification technique would be suitable for use in in vitro maturation procedures.
As in Keros et al21, we found a small and constant percentage of atretic follicles in our study, suggesting that some structures might not have been recognized as follicles after atresia. Analyzing the transitional stage of development, the same study found a well-conserved viability using slow freezing with three types of cryoprotectants (propanodiol, ethylene glycol, and sucrose). In our study, we used a combination of two cryoprotectants (ethylene glycol and sucrose), and did not observe this conservation. This suggests that the use of a greater number of cryoprotectants could increase the maintenance of follicular viability, especially in follicles with different cellular structures, such as transient follicles.17 Differences may not have been seen between the primary, secondary, and antral follicles due to the small number of follicles found in these stages of development.
In this study, a combination of glycerol and ethylene glycol was chosen for vitrification, and a combination of sucrose and ethylene glycol was chosen for slow freezing. These choices were based on successful results previously obtained for the preservation of tissue integrity and functionality.32 In vitrification, a minimum volume of solution is used between the fragments, for this method showed good results in oocytes and embryos, as it improves the cooling rate and reduces the toxicity of cryoprotectants because its concentration is already quite high.33
Growing follicles for 7 days after thawing was an effective way to support the results of the morphological findings. This is due to the fact that the decrease in the number of primordial follicles and the significant increase in the number of transient follicles with both methods suggested that the follicles were able to develop in the proposed culture. This same finding was observed in all stages of follicle development. Although not significant, the results were promising, but we must highlight again that both techniques were effective. Our findings are consistent with those of a previously published study, where after only 2 days in culture, the number of primordial follicles drastically decreased. In parallel, an increase in the number of follicles in the initial primary stage was found. This also suggested the activation capacity and growth of follicles in the culture medium.34
The slow freezing technique has already produced 60 live births after retransplantation. For this reason, many clinics adopt it as the standard cryopreservation method for ovarian tissue. The results of this study showed that both vitrification and slow freezing were able to morphologically conserve the follicular structures during the various stages of development.
Vitrification is a fast method, and does not require any equipment for programming the freezing curve. Therefore, it may be a more practical method to apply in a laboratory routine. For these reasons, it allows for the cryopreservation of larger numbers of samples, which will increase the chances of hormonal maintenance and future pregnancy.
Despite the limitation of not being able to freeze the same fragment using both techniques, and difference in the number of follicles in the fragments, even using the same cow ovary, our study showed no significant difference between the two protocols. Despite the significant loss in the number of follicles after vitrification and slow freezing, both techniques were able to maintain a large number of viable follicles after heating, with no difference between them in the tissue morphological assessment. The ovarian tissue fragments maintained their ability to develop in the culture medium in both methods.
As no significant differences were found between the protocols, vitrification may be considered as an advance and an alternative cryopreservation method for ovarian tissue in patients who will undergo treatments that can lead to ovarian failure, as the method is cheaper, faster, and better adaptable to the routine of an in vitro fertilization laboratory. This will allow more ovarian tissue fragments to be cryopreserved and, as a result, it will increase the chances of a future pregnancy or hormonal recovery in these patients.
References
- 1.Lamar C A, DeCherney A H. Fertility preservation: state of the science and future research directions. Fertil Steril. 2009;91(2):316–319. doi: 10.1016/j.fertnstert.2008.08.133. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho B R, Rodrigues J K, Campos J R, Marinho R M, Caetano J PJ, Rosa-e-Silva A CJS. Visão geral sobre preservação da fertilidade feminina depois do câncer. Reprod Clim. 2014;29(3):123–129. [Google Scholar]
- 3.Brasil Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva [Internet] Rio de Janeiro: INCA; 2014 [citado 2015 Out 23]. Disponível em: http://www2.inca.gov.br/wps/wcm/connect/inca/portal/home [Google Scholar]
- 4.Donnez J, Bassil S. Indications for cryopreservation of ovarian tissue. Hum Reprod Update. 1998;4(3):248–259. doi: 10.1093/humupd/4.3.248. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F Loibl S Pagani O, et al; European Society of Breast Cancer Specialists. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer Eur J Cancer 201248183355–3377. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Dolmans M M. Preservation of fertility in females with haematological malignancy. Br J Haematol. 2011;154(2):175–184. doi: 10.1111/j.1365-2141.2011.08723.x. [DOI] [PubMed] [Google Scholar]
- 7.Kondapalli L A Ovarian tissue cryopreservation and transplantation New York: Springer; 2012. p. 63–75. [Google Scholar]
- 8.Grynberg M Poulain M Le Parco S Sebag-Peyrelevade S Frydman N Benachi A [How to preserve female fertility before cancer treatments?]. [in French]Rev Prat 2013633314–318. [PubMed] [Google Scholar]
- 9.Anderson R A, McLaughlin M, Wallace W H, Albertini D F, Telfer E E. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29(1):97–106. doi: 10.1093/humrep/det388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnez J, Dolmans M M. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–1170. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marinho R M Rodrigues J K Lamaita R M, et al. Preservação da fertilidade em mulheres com câncer: atualização e perspectivas Rev Med Minas Gerais. 2013234510–517. [Google Scholar]
- 12.Donnez J, Dolmans M M. Ovarian tissue freezing: current status. Curr Opin Obstet Gynecol. 2015;27(3):222–230. doi: 10.1097/GCO.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 13.Cobo A, Domingo J, Pérez S, Crespo J, Remohí J, Pellicer A. Vitrification: an effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol. 2008;10(5):268–273. doi: 10.1007/s12094-008-0196-7. [DOI] [PubMed] [Google Scholar]
- 14.AbdelHafez F F, Desai N, Abou-Setta A M, Falcone T, Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online. 2010;20(2):209–222. doi: 10.1016/j.rbmo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini T A, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006;85 01:1150–1156. doi: 10.1016/j.fertnstert.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Mathias F J, D'Souza F, Uppangala S, Salian S R, Kalthur G, Adiga S K. Ovarian tissue vitrification is more efficient than slow freezing in protecting oocyte and granulosa cell DNA integrity. Syst Biol Reprod Med. 2014;60(6):317–322. doi: 10.3109/19396368.2014.923542. [DOI] [PubMed] [Google Scholar]
- 17.Klocke S, Bündgen N, Köster F, Eichenlaub-Ritter U, Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet. 2015;291(2):419–426. doi: 10.1007/s00404-014-3390-6. [DOI] [PubMed] [Google Scholar]
- 18.Yeoman R R, Wolf D P, Lee D M. Coculture of monkey ovarian tissue increases survival after vitrification and slow-rate freezing. Fertil Steril. 2005;83 01:1248–1254. doi: 10.1016/j.fertnstert.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Ting A Y, Yeoman R R, Lawson M S, Zelinski M B. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26(9):2461–2472. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Oncofertility Consortium National Physicians Cooperative [Internet]. Chicago: Oncofertility Consortium; 2015 [cited 2015 Sep 10] Available from: http://oncofertility.northwestern.edu/resources/national-physicians-cooperative
- 21.Keros V Xella S Hultenby K, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue Hum Reprod 20092471670–1683. [DOI] [PubMed] [Google Scholar]
- 22.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18(4):568–577. doi: 10.1016/s1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- 23.Hovatta O. Methods for cryopreservation of human ovarian tissue. Reprod Biomed Online. 2005;10(6):729–734. doi: 10.1016/s1472-6483(10)61116-9. [DOI] [PubMed] [Google Scholar]
- 24.Harp R, Leibach J, Black J, Keldahl C, Karow A. Cryopreservation of murine ovarian tissue. Cryobiology. 1994;31(4):336–343. doi: 10.1006/cryo.1994.1040. [DOI] [PubMed] [Google Scholar]
- 25.Herraiz S Novella-Maestre E Rodríguez B, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices Fertil Steril 20141013775–784. [DOI] [PubMed] [Google Scholar]
- 26.Sanfilippo S Canis M Smitz J, et al. Vitrification of human ovarian tissue: a practical and relevant alternative to slow freezing Reprod Biol Endocrinol 20151367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos S S Ferreira M A Pinto J A, et al. Characterization of folliculogenesis and the occurrence of apoptosis in the development of the bovine fetal ovary Theriogenology 2013792344–350. [DOI] [PubMed] [Google Scholar]
- 28.Kim S S, Lee W S, Chung M K, Lee H C, Lee H H, Hill D. Long-term ovarian function and fertility after heterotopic autotransplantation of cryobanked human ovarian tissue: 8-year experience in cancer patients. Fertil Steril. 2009;91(6):2349–2354. doi: 10.1016/j.fertnstert.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues J K Campos J R Marinho R M Zelinski M B Stouffer R L Xu J Desenvolvimento folicular e maturação oocitária in vitro Rio de Janeiro: Medbook; 2015. p. 161–9. [Google Scholar]
- 30.Telfer E E, McLaughlin M, Ding C, Thong K J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues J K, Navarro P A, Zelinski M B, Stouffer R L, Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Müllerian hormone by individual macaque follicles during three-dimensional culture. Hum Reprod. 2015;30(3):664–674. doi: 10.1093/humrep/deu335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanpradit N, Comizzoli P, Srisuwatanasagul S, Chatdarong K. Positive impact of sucrose supplementation during slow freezing of cat ovarian tissues on cellular viability, follicle morphology, and DNA integrity. Theriogenology. 2015;83(9):1553–1561. doi: 10.1016/j.theriogenology.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Dos Santos Neto P C, Vilariño M, Barrera N, Cuadro F, Crispo M, Menchaca A. Cryotolerance of Day 2 or Day 6 in vitro produced ovine embryos after vitrification by Cryotop or Spatula methods. Cryobiology. 2015;70(1):17–22. doi: 10.1016/j.cryobiol.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Wandji S A, Srsen V, Nathanielsz P W, Eppig J J, Fortune J E. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12(9):1993–2001. doi: 10.1093/humrep/12.9.1993. [DOI] [PubMed] [Google Scholar]


